Abstract
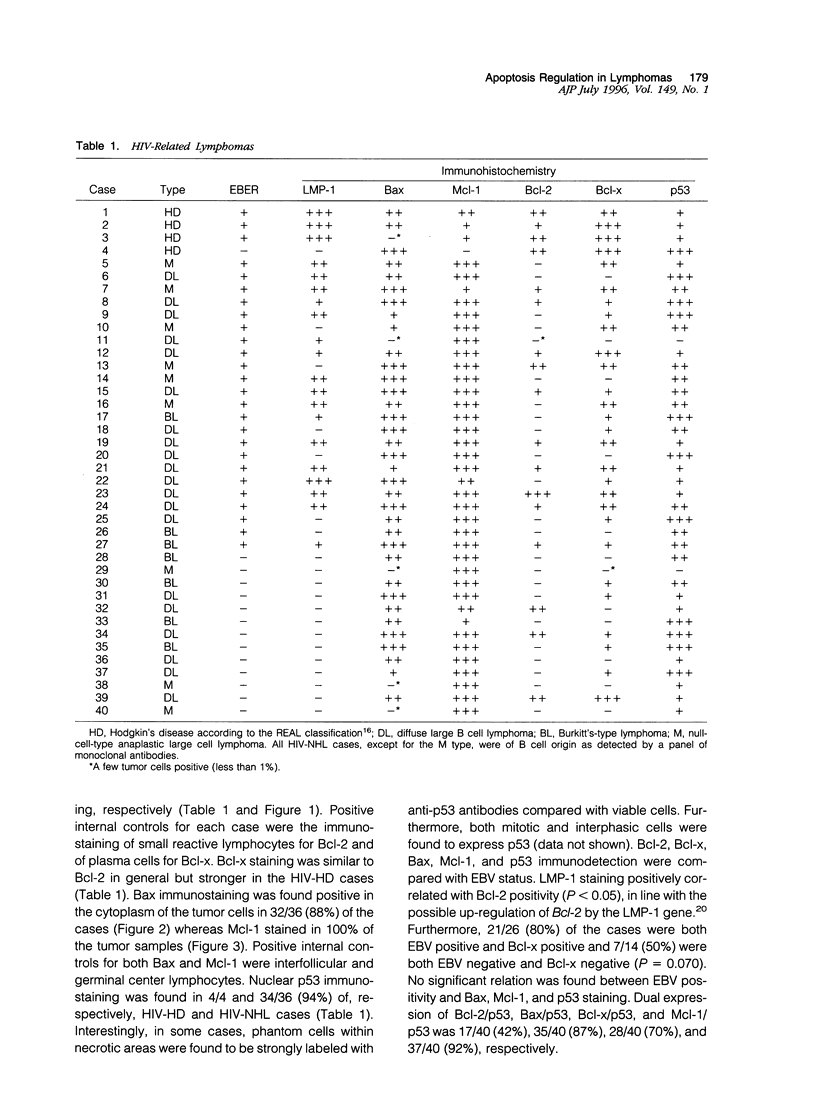
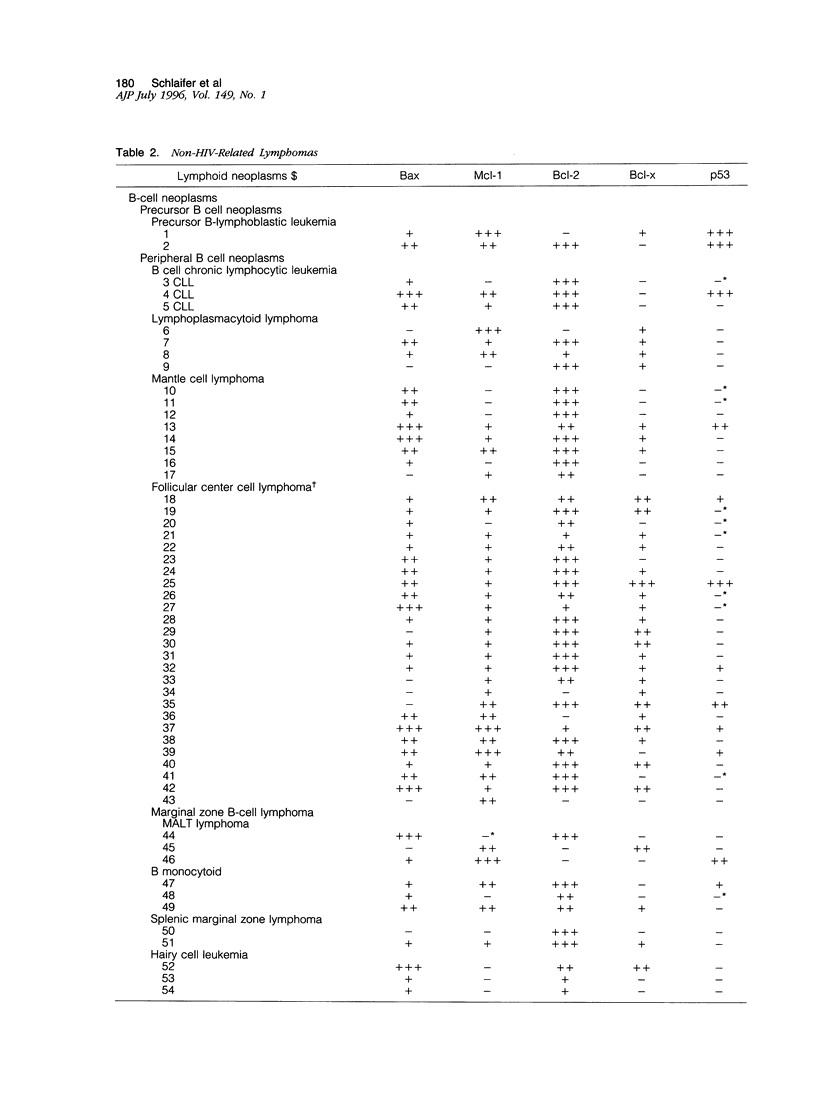
The expression of the apoptosis-regulating genes Bcl-2, Bcl-x, Bax, Mcl-1, and p53 analyzed in 4 cases of human immunodeficiency virus (HIV)-associated Hodgkin's disease, in 36 cases of HIV-related non-Hodgkin's lymphomas (NHLs), and in 109 cases of non-HIV-related NHLs by using immunohistochemistry. HIV-associated Hodgkin's disease samples were positive for all markers. For the HIV-related NHL samples, 36, 66, 88, 100, and 94% of the cases were Bcl-2, Bcl-x, Bax, Mcl-1, and p53 were found to be expressed in 69, 65, 82, 83, and 42%, respectively. No significant differences were observed in Bax and Mcl-1 staining between HIV-unrelated NHLs of B cell and T cell types. In contrast, Bcl-2 was positive in 66/79 (83%) and 10/30 (33%) of B cell and T cell HIV-unrelated NHLs, respectively (P2 < 0.001). Peculiar patterns were observed for hairy cell leukemia (Bax+, Bcl-2+, Mcl-1-) and for anaplastic large cell lymphoma (Bax+, Mcl-1+, Bcl-2-) in HIV-unrelated NHLs. Of interest, all cases with a positive expression of Bax were also found to express either Mcl-1 and/or Bcl-2, suggesting that Mcl-1 and Bcl-2 may counteract the pro-apoptosis function of Bax in vivo by protein-protein interaction within the tumor cell, as demonstrated previously in vitro. These results suggest that apoptosis regulation may have a role in the pathogenesis of some HIV-related and HIV-unrelated NHLs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Boyd J. M., Malstrom S., Subramanian T., Venkatesh L. K., Schaeper U., Elangovan B., D'Sa-Eipper C., Chinnadurai G. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994 Oct 21;79(2):341–351. doi: 10.1016/0092-8674(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Brousset P., Butet V., Chittal S., Selves J., Delsol G. Comparison of in situ hybridization using different nonisotopic probes for detection of Epstein-Barr virus in nasopharyngeal carcinoma and immunohistochemical correlation with anti-latent membrane protein antibody. Lab Invest. 1992 Oct;67(4):457–464. [PubMed] [Google Scholar]
- Chittenden T., Harrington E. A., O'Connor R., Flemington C., Lutz R. J., Evan G. I., Guild B. C. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Hamilton-Dutoit S. J., Raphael M., Audouin J., Diebold J., Lisse I., Pedersen C., Oksenhendler E., Marelle L., Pallesen G. In situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in acquired immunodeficiency syndrome-related lymphomas: correlation with tumor morphology and primary site. Blood. 1993 Jul 15;82(2):619–624. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Imamura J., Miyoshi I., Koeffler H. P. p53 in hematologic malignancies. Blood. 1994 Oct 15;84(8):2412–2421. [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Blomqvist C., Franssila K., Krajewska M., Wasenius V. M., Niskanen E., Nordling S., Reed J. C. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995 Oct 1;55(19):4471–4478. [PubMed] [Google Scholar]
- Krajewski S., Bodrug S., Gascoyne R., Berean K., Krajewska M., Reed J. C. Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol. 1994 Sep;145(3):515–525. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Wang H. G., Irie S., Fong L., Reed J. C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994 Nov 1;54(21):5501–5507. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Said J. W., Miller C. W., Koeffler H. P. Mutation and protein expression of p53 in acquired immunodeficiency syndrome-related lymphomas. Blood. 1993 Aug 1;82(3):920–926. [PubMed] [Google Scholar]
- Oltvai Z. N., Korsmeyer S. J. Checkpoints of dueling dimers foil death wishes. Cell. 1994 Oct 21;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Gatter K. What is the value of bcl-2 protein detection for histopathologists? Histopathology. 1995 Jan;26(1):89–93. doi: 10.1111/j.1365-2559.1995.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Prokocimer M., Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994 Oct 15;84(8):2391–2411. [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. E., Yang T., Qian L., Jenkinson J. D., Zhou P., Eastman A., Craig R. W. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994 Dec 15;54(24):6348–6352. [PubMed] [Google Scholar]
- Schlaifer D., Brousset P., Attal M., Massip P., Payen C., Marchou B., Huguet F., Muller C., Laurent G., Pris J. bcl-2 proto-oncogene and Epstein-Barr virus latent membrane protein-1 expression in AIDS-related lymphoma. Histopathology. 1994 Jul;25(1):77–82. doi: 10.1111/j.1365-2559.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Schlaifer D., March M., Krajewski S., Laurent G., Pris J., Delsol G., Reed J. C., Brousset P. High expression of the bcl-x gene in Reed-Sternberg cells of Hodgkin's disease. Blood. 1995 May 15;85(10):2671–2674. [PubMed] [Google Scholar]
- Sinicrope F. A., Ruan S. B., Cleary K. R., Stephens L. C., Lee J. J., Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995 Jan 15;55(2):237–241. [PubMed] [Google Scholar]
- Takayama S., Sato T., Krajewski S., Kochel K., Irie S., Millan J. A., Reed J. C. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995 Jan 27;80(2):279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995 Jan 27;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]







