Abstract
Inflammation is a critical feature of atherosclerosis and is characterized in part by the migration of circulating monocytes to the atherosclerotic plaque. These monocytes, together with macrophages, are a source of cytokines, growth factors, proteases, and procoagulants, which contribute to the progression of the atherosclerosis lesion. This study employed a modified Boyden chamber to examine the secretion of monocyte chemotactic activity by cultured rat aortic vascular smooth muscle cells in response to growth factors and cytokines. The induction of monocyte chemotactic activity showed a surprising specificity for platelet-derived growth factor-BB. This activity was blocked by actinomycin D and cycloheximide and thus required de novo transcription and protein synthesis. The ability to stimulate monocyte migration appeared to be solely due to the secretion of the monocyte chemoattractant protein JE/MCP-1 and was completely blocked by antisense oligonucleotides and antibodies to JE/MCP-1. The induction of chemotactic activity was also blocked by dexamethasone, an inhibitor of JE mRNA accumulation. This study suggests that the secretion of monocyte chemotactic activity by vascular smooth muscle cells is a highly regulatable and specific event and underscores the importance of JE/MCP-1 in the inflammatory response of the vessel wall.
Full text
PDF


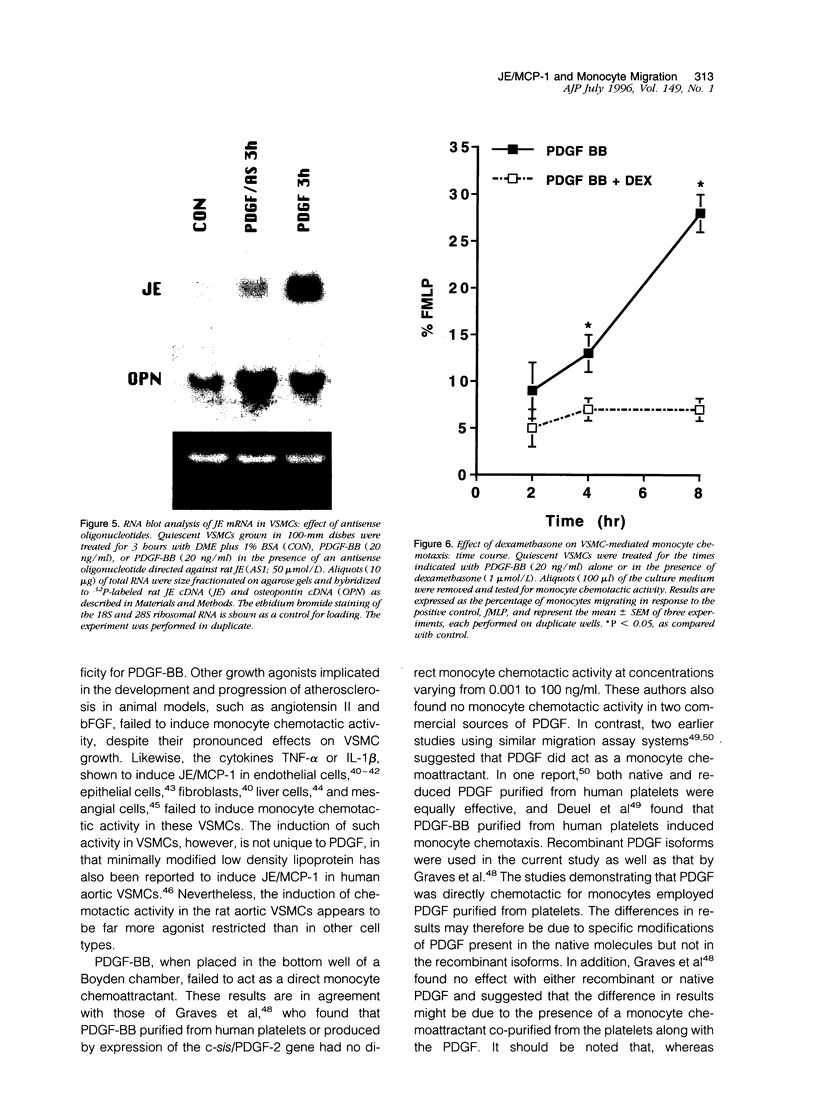
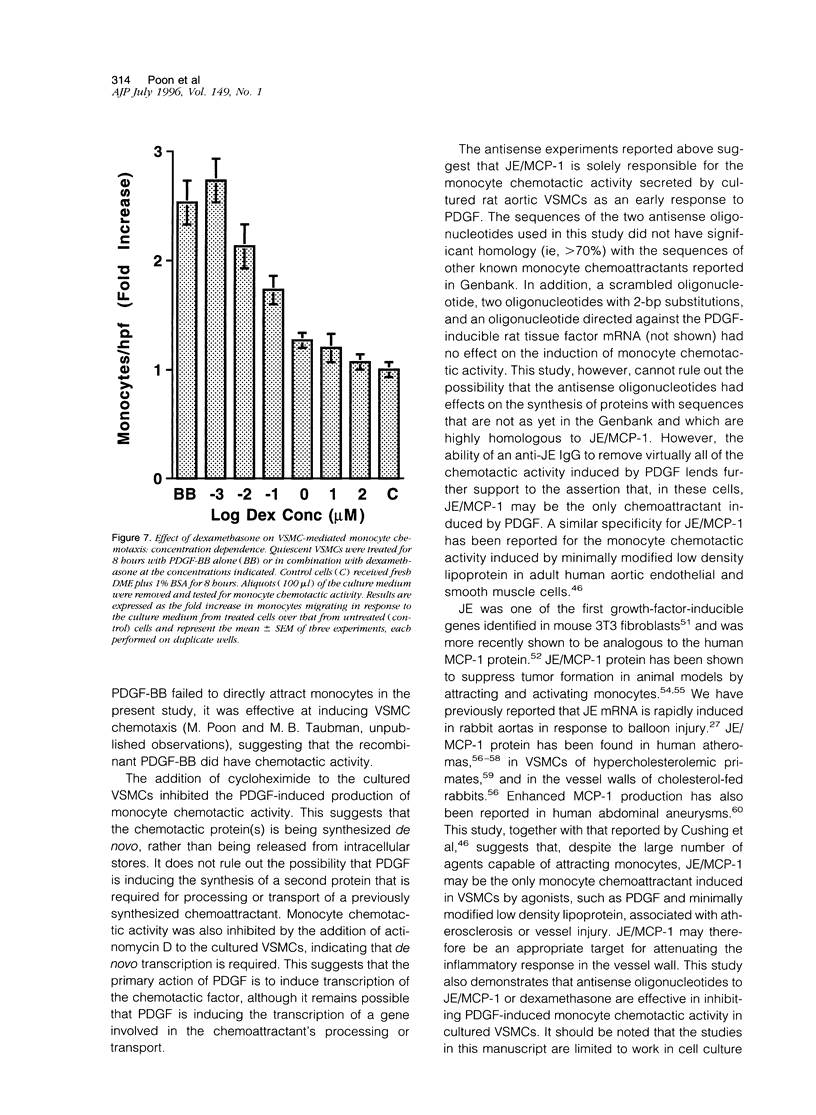



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985 Jul;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Vekshtein V., Gordon H. M., Tsuda T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989 Apr;13(4):305–314. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Libby P. Cytokines and growth factors in atherogenesis. Arch Pathol Lab Med. 1992 Dec;116(12):1292–1300. [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Randolph G. J. Chemokines and tissue injury. Am J Pathol. 1995 Jun;146(6):1287–1301. [PMC free article] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Goss J. A., Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985 Jan-Feb;5(1):55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Graves D. T., Grotendorst G. R., Antoniades H. N., Schwartz C. J., Valente A. J. Platelet-derived growth factor is not chemotactic for human peripheral blood monocytes. Exp Cell Res. 1989 Feb;180(2):497–503. doi: 10.1016/0014-4827(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Green R. S., Lieb M. E., Weintraub A. S., Gacheru S. N., Rosenfield C. L., Shah S., Kagan H. M., Taubman M. B. Identification of lysyl oxidase and other platelet-derived growth factor-inducible genes in vascular smooth muscle cells by differential screening. Lab Invest. 1995 Oct;73(4):476–482. [PubMed] [Google Scholar]
- Inui H., Kitami Y., Kondo T., Inagami T. Transduction of mitogenic activity of platelet-derived growth factor (PDGF) AB by PDGF-beta receptor without participation of PDGF-alpha receptor in vascular smooth muscle cells. J Biol Chem. 1993 Aug 15;268(23):17045–17050. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kappelmayer J., Bernabei A., Edmunds L. H., Jr, Edgington T. S., Colman R. W. Tissue factor is expressed on monocytes during simulated extracorporeal circulation. Circ Res. 1993 May;72(5):1075–1081. doi: 10.1161/01.res.72.5.1075. [DOI] [PubMed] [Google Scholar]
- Klurfeld D. M. Identification of foam cells in human atherosclerotic lesions as macrophages using monoclonal antibodies. Arch Pathol Lab Med. 1985 May;109(5):445–449. [PubMed] [Google Scholar]
- Koch A. E., Haines G. K., Rizzo R. J., Radosevich J. A., Pope R. M., Robinson P. G., Pearce W. H. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990 Nov;137(5):1199–1213. [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Pearce W. H., Shah M. R., Parikh D., Evanoff H. L., Haines G. K., Burdick M. D., Strieter R. M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993 May;142(5):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Konishi F., Inui H., Inagami T. Differing signal transductions elicited by three isoforms of platelet-derived growth factor in vascular smooth muscle cells. J Biol Chem. 1993 Feb 25;268(6):4458–4464. [PubMed] [Google Scholar]
- Koyama N., Hart C. E., Clowes A. W. Different functions of the platelet-derived growth factor-alpha and -beta receptors for the migration and proliferation of cultured baboon smooth muscle cells. Circ Res. 1994 Oct;75(4):682–691. doi: 10.1161/01.res.75.4.682. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Marra F., Valente A. J., Pinzani M., Abboud H. E. Cultured human liver fat-storing cells produce monocyte chemotactic protein-1. Regulation by proinflammatory cytokines. J Clin Invest. 1993 Oct;92(4):1674–1680. doi: 10.1172/JCI116753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier V., Houssaye S., Ternisien C., Léon A., de Verneuil H., Elbim C., Mackman N., Edgington T. S., de Prost D. Endotoxin-induced tissue factor messenger RNA in human monocytes is negatively regulated by a cyclic AMP-dependent mechanism. Blood. 1993 Feb 15;81(4):973–979. [PubMed] [Google Scholar]
- Poon M., Megyesi J., Green R. S., Zhang H., Rollins B. J., Safirstein R., Taubman M. B. In vivo and in vitro inhibition of JE gene expression by glucocorticoids. J Biol Chem. 1991 Nov 25;266(33):22375–22379. [PubMed] [Google Scholar]
- Rogers C., Karnovsky M. J., Edelman E. R. Inhibition of experimental neointimal hyperplasia and thrombosis depends on the type of vascular injury and the site of drug administration. Circulation. 1993 Sep;88(3):1215–1221. doi: 10.1161/01.cir.88.3.1215. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Walz A., Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991 Aug 15;78(4):1112–1116. [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sachinidis A., Schulte K., Ko Y., Meyer zu Brickwedde M. K., Hoppe V., Hoppe J., Vetter H. The induction of early response genes in rat smooth muscle cells by PDGF-AA is not sufficient to stimulate DNA-synthesis. FEBS Lett. 1993 Mar 22;319(3):221–224. doi: 10.1016/0014-5793(93)80550-e. [DOI] [PubMed] [Google Scholar]
- Satriano J. A., Shuldiner M., Hora K., Xing Y., Shan Z., Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest. 1993 Sep;92(3):1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Wang J. M., Colotta F., Dejana E., Mantovani A., Oppenheim J. J., Larsen C. G., Zachariae C. O., Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990 Apr 15;144(8):3034–3038. [PubMed] [Google Scholar]
- St Clair R. W., Randolph R. K., Jokinen M. P., Clarkson T. B., Barakat H. A. Relationship of plasma lipoproteins and the monocyte-macrophage system to atherosclerosis severity in cholesterol-fed pigeons. Arteriosclerosis. 1986 Nov-Dec;6(6):614–626. doi: 10.1161/01.atv.6.6.614. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Phan S. H., Rollins B. J., Strieter R. M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991 May 25;266(15):9912–9918. [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Takeya M., Yoshimura T., Leonard E. J., Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993 May;24(5):534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Rollins B. J., Poon M., Marmur J., Green R. S., Berk B. C., Nadal-Ginard B. JE mRNA accumulates rapidly in aortic injury and in platelet-derived growth factor-stimulated vascular smooth muscle cells. Circ Res. 1992 Feb;70(2):314–325. doi: 10.1161/01.res.70.2.314. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Vedeler C. A., Nyland H., Matre R. In situ characterization of the foam cells in early human atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand C. 1984 Apr;92(2):133–137. doi: 10.1111/j.1699-0463.1984.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- Wiseman D. M., Polverini P. J., Kamp D. W., Leibovich S. J. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988 Dec 15;157(2):793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol. 1990 Mar 15;144(6):2377–2383. [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Tanaka S., Appella E., Kuratsu J., Leonard E. J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Dluz S., Graves D. T., Zhang L., Antoniades H. N., Hollander W., Prusty S., Valente A. J., Schwartz C. J., Sonenshein G. E. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6953–6957. doi: 10.1073/pnas.89.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusekom H. M., van der Giessen W. J., van Suylen R., Bos E., Bosman F. T., Serruys P. W. Histology after stenting of human saphenous vein bypass grafts: observations from surgically excised grafts 3 to 320 days after stent implantation. J Am Coll Cardiol. 1993 Jan;21(1):45–54. doi: 10.1016/0735-1097(93)90715-d. [DOI] [PubMed] [Google Scholar]