Abstract
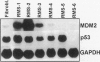
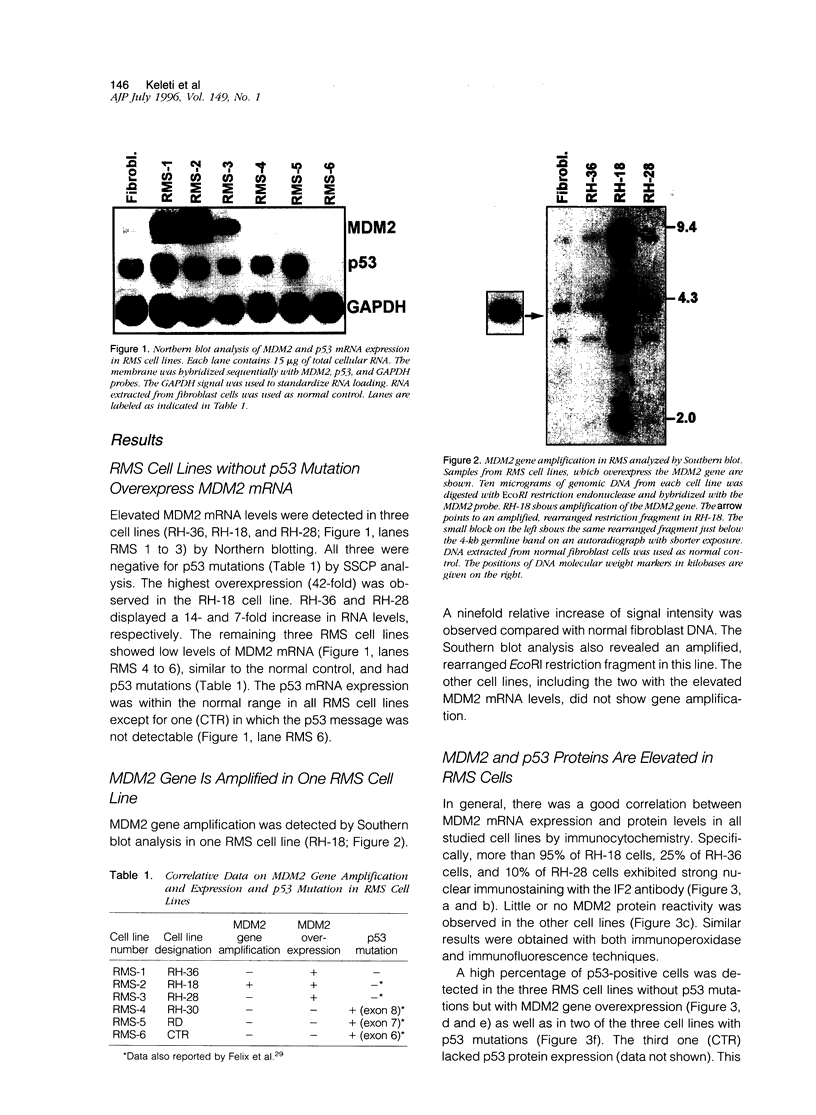
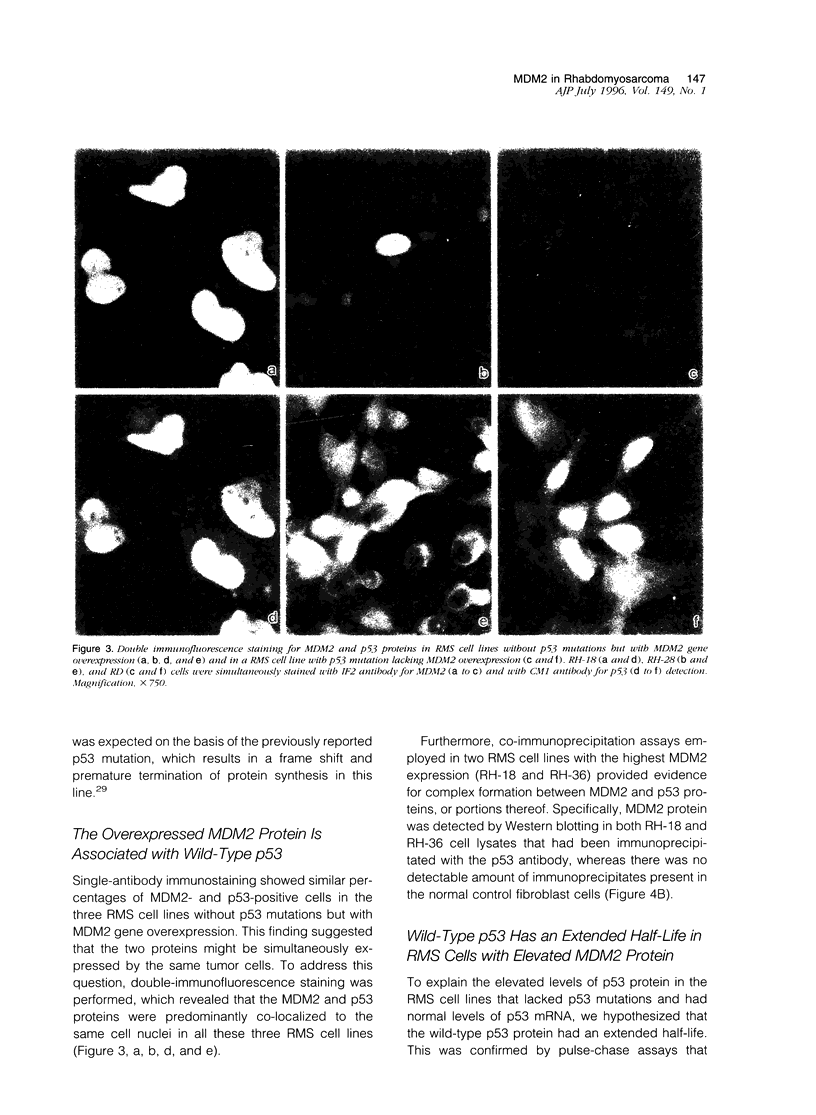
MDM2 gene overexpression has been implicated in the pathogenesis of human neoplasia via inhibition of the p53 tumor-suppressor function. To investigate the potential involvement of the MDM2 oncogene in the pathogenesis of childhood rhabdomyosarcoma (RMS) we studied MDM2 abnormalities in six RMS cell lines in correlation with the p53 status. Three showed overexpression of MDM2 mRNA and protein, one with concomitant MDM2 gene amplification. All three lacked p53 mutation and expressed low levels of p53 mRNA but exhibited elevated p53 proteins. Double immunostaining revealed that the overexpressed MDM2 and p53 proteins were co-localized to the same cell nuclei. Furthermore, the two proteins were physically associated, as shown by co-immunoprecipitation and Western blot analysis. The half-life of the p53 protein was prolonged in the MDM2-expressing RMS cells. The extended half-life wildtype p53 protein and its complex formation with the elevated MDM2 suggest that the underlying mechanism for p53 protein accumulation in these cell lines is p53 stabilization by an overabundant MDM2 protein. The overexpressed MDM2 protein had a short half-life. The three remaining RMS cell lines exhibited low MDM2 mRNA and protein levels and carried p53 mutations. This study suggest that MDM2 overexpression represents an alternative mechanism for p53 inactivation in a subset of childhood RMS without p53 mutations. The results further indicate that the elevated MDM2 protein is responsible for wildtype p53 protein accumulation via stabilization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesina A. M., Nalbantoglu J., Cavenee W. K. p53 gene mutation and mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res. 1994 Nov 1;54(21):5649–5651. [PubMed] [Google Scholar]
- Barak Y., Juven T., Haffner R., Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J. 1993 Feb;12(2):461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N., Falkoff D., Petruzzi M. J., Nowak N., Zabel B., Adam M., Aguiar M. C., Grundy P., Shows T., Pelletier J. Anaplastic Wilms' tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet. 1994 May;7(1):91–97. doi: 10.1038/ng0594-91. [DOI] [PubMed] [Google Scholar]
- Bueso-Ramos C. E., Yang Y., deLeon E., McCown P., Stass S. A., Albitar M. The human MDM-2 oncogene is overexpressed in leukemias. Blood. 1993 Nov 1;82(9):2617–2623. [PubMed] [Google Scholar]
- Chang F., Syrjänen S., Tervahauta A., Syrjänen K. Tumourigenesis associated with the p53 tumour suppressor gene. Br J Cancer. 1993 Oct;68(4):653–661. doi: 10.1038/bjc.1993.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Latres E., Drobnjak M., Oliva M. R., Pollack D., Woodruff J. M., Marechal V., Chen J., Brennan M. F., Levine A. J. Molecular abnormalities of mdm2 and p53 genes in adult soft tissue sarcomas. Cancer Res. 1994 Feb 1;54(3):794–799. [PubMed] [Google Scholar]
- Fakharzadeh S. S., Trusko S. P., George D. L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991 Jun;10(6):1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix C. A., Kappel C. C., Mitsudomi T., Nau M. M., Tsokos M., Crouch G. D., Nisen P. D., Winick N. J., Helman L. J. Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res. 1992 Apr 15;52(8):2243–2247. [PubMed] [Google Scholar]
- Finlay C. A. The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol Cell Biol. 1993 Jan;13(1):301–306. doi: 10.1128/mcb.13.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flørenes V. A., Maelandsmo G. M., Forus A., Andreassen A., Myklebost O., Fodstad O. MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst. 1994 Sep 7;86(17):1297–1302. doi: 10.1093/jnci/86.17.1297. [DOI] [PubMed] [Google Scholar]
- Gaidano G., Ballerini P., Gong J. Z., Inghirami G., Neri A., Newcomb E. W., Magrath I. T., Knowles D. M., Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi T., Kinoshita H., Yamada H., Kakehi Y., Ogawa O., Wu W. J., Takahashi R., Sugiyama T., Yoshida O. Oncogene amplification in urothelial cancers with p53 gene mutation or MDM2 amplification. J Natl Cancer Inst. 1994 Sep 7;86(17):1331–1335. doi: 10.1093/jnci/86.17.1331. [DOI] [PubMed] [Google Scholar]
- Haines D. S., Landers J. E., Engle L. J., George D. L. Physical and functional interaction between wild-type p53 and mdm2 proteins. Mol Cell Biol. 1994 Feb;14(2):1171–1178. doi: 10.1128/mcb.14.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Q., Raphael B., Buchbinder A., Li J. J., Zhang W. G., Friedman-Kien A. E. Rearrangement and expression of MDM2 oncogene in chronic lymphocytic leukemia. Am J Hematol. 1994 Oct;47(2):139–141. doi: 10.1002/ajh.2830470215. [DOI] [PubMed] [Google Scholar]
- Kovar H., Auinger A., Jug G., Aryee D., Zoubek A., Salzer-Kuntschik M., Gadner H. Narrow spectrum of infrequent p53 mutations and absence of MDM2 amplification in Ewing tumours. Oncogene. 1993 Oct;8(10):2683–2690. [PubMed] [Google Scholar]
- Ladanyi M., Cha C., Lewis R., Jhanwar S. C., Huvos A. G., Healey J. H. MDM2 gene amplification in metastatic osteosarcoma. Cancer Res. 1993 Jan 1;53(1):16–18. [PubMed] [Google Scholar]
- Ladanyi M., Lewis R., Jhanwar S. C., Gerald W., Huvos A. G., Healey J. H. MDM2 and CDK4 gene amplification in Ewing's sarcoma. J Pathol. 1995 Feb;175(2):211–217. doi: 10.1002/path.1711750209. [DOI] [PubMed] [Google Scholar]
- Landers J. E., Haines D. S., Strauss J. F., 3rd, George D. L. Enhanced translation: a novel mechanism of mdm2 oncogene overexpression identified in human tumor cells. Oncogene. 1994 Sep;9(9):2745–2750. [PubMed] [Google Scholar]
- Leach F. S., Tokino T., Meltzer P., Burrell M., Oliner J. D., Smith S., Hill D. E., Sidransky D., Kinzler K. W., Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993 May 15;53(10 Suppl):2231–2234. [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr, Mulvihill J. J., Blattner W. A., Dreyfus M. G., Tucker M. A., Miller R. W. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988 Sep 15;48(18):5358–5362. [PubMed] [Google Scholar]
- Lianes P., Orlow I., Zhang Z. F., Oliva M. R., Sarkis A. S., Reuter V. E., Cordon-Cardo C. Altered patterns of MDM2 and TP53 expression in human bladder cancer. J Natl Cancer Inst. 1994 Sep 7;86(17):1325–1330. doi: 10.1093/jnci/86.17.1325. [DOI] [PubMed] [Google Scholar]
- Lin J., Chen J., Elenbaas B., Levine A. J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994 May 15;8(10):1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- Lipford E. H., Smith H. R., Pittaluga S., Jaffe E. S., Steinberg A. D., Cossman J. Clonality of angioimmunoblastic lymphadenopathy and implications for its evolution to malignant lymphoma. J Clin Invest. 1987 Feb;79(2):637–642. doi: 10.1172/JCI112860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro R., Gloghini A., Doglioni C., Gasparotto D., Vukosavljevic T., De Re V., Laurino L., Carbone A., Boiocchi M. MDM2 overexpression does not account for stabilization of wild-type p53 protein in non-Hodgkin's lymphomas. Blood. 1995 Jun 1;85(11):3239–3246. [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mietz J. A., Unger T., Huibregtse J. M., Howley P. M. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992 Dec;11(13):5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Matlashewski G. J., Scrable H. J., Cavenee W. K. Mechanisms of p53 loss in human sarcomas. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5863–5867. doi: 10.1073/pnas.87.15.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Pietenpol J. A., Thiagalingam S., Gyuris J., Kinzler K. W., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993 Apr 29;362(6423):857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Olson D. C., Marechal V., Momand J., Chen J., Romocki C., Levine A. J. Identification and characterization of multiple mdm-2 proteins and mdm-2-p53 protein complexes. Oncogene. 1993 Sep;8(9):2353–2360. [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Perry M. E., Piette J., Zawadzki J. A., Harvey D., Levine A. J. The mdm-2 gene is induced in response to UV light in a p53-dependent manner. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11623–11627. doi: 10.1073/pnas.90.24.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberger G., Liu L., Ichimura K., Schmidt E. E., Collins V. P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993 Jun 15;53(12):2736–2739. [PubMed] [Google Scholar]
- Reihsaus E., Kohler M., Kraiss S., Oren M., Montenarh M. Regulation of the level of the oncoprotein p53 in non-transformed and transformed cells. Oncogene. 1990 Jan;5(1):137–145. [PubMed] [Google Scholar]
- Riou G., Barrois M., Prost S., Terrier M. J., Theodore C., Levine A. J. The p53 and mdm-2 genes in human testicular germ-cell tumors. Mol Carcinog. 1995 Mar;12(3):124–131. doi: 10.1002/mc.2940120303. [DOI] [PubMed] [Google Scholar]
- Rogel A., Popliker M., Webb C. G., Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985 Oct;5(10):2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovinski B., Benchimol S. Immortalization of rat embryo fibroblasts by the cellular p53 oncogene. Oncogene. 1988 May;2(5):445–452. [PubMed] [Google Scholar]
- Santibáez-Koref M. F., Birch J. M., Hartley A. L., Jones P. H., Craft A. W., Eden T., Crowther D., Kelsey A. M., Harris M. p53 germline mutations in Li-Fraumeni syndrome. Lancet. 1991 Dec 14;338(8781):1490–1491. doi: 10.1016/0140-6736(91)92303-j. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Sheikh M. S., Shao Z. M., Hussain A., Fontana J. A. The p53-binding protein MDM2 gene is differentially expressed in human breast carcinoma. Cancer Res. 1993 Jul 15;53(14):3226–3228. [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber P. G., Chen J., Nisen P. D. Infrequency of MDM2 gene amplification in pediatric solid tumors and lack of association with p53 mutations in adult squamous cell carcinomas. Cancer Res. 1993 Dec 15;53(24):6028–6030. [PubMed] [Google Scholar]
- Whang-Peng J., Knutsen T., Theil K., Horowitz M. E., Triche T. Cytogenetic studies in subgroups of rhabdomyosarcoma. Genes Chromosomes Cancer. 1992 Nov;5(4):299–310. doi: 10.1002/gcc.2870050405. [DOI] [PubMed] [Google Scholar]
- Wu X., Bayle J. H., Olson D., Levine A. J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993 Jul;7(7A):1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Yew P. R., Berk A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992 May 7;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Bienz-Tadmor B., Givol D., Oren M. Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells. EMBO J. 1985 May;4(5):1251–1255. doi: 10.1002/j.1460-2075.1985.tb03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman A., Barak Y., Ragimov N., Levy N., Oren M. Sequence-specific DNA binding by p53: identification of target sites and lack of binding to p53 - MDM2 complexes. EMBO J. 1993 Jul;12(7):2799–2808. doi: 10.1002/j.1460-2075.1993.tb05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]