Abstract
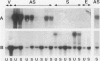
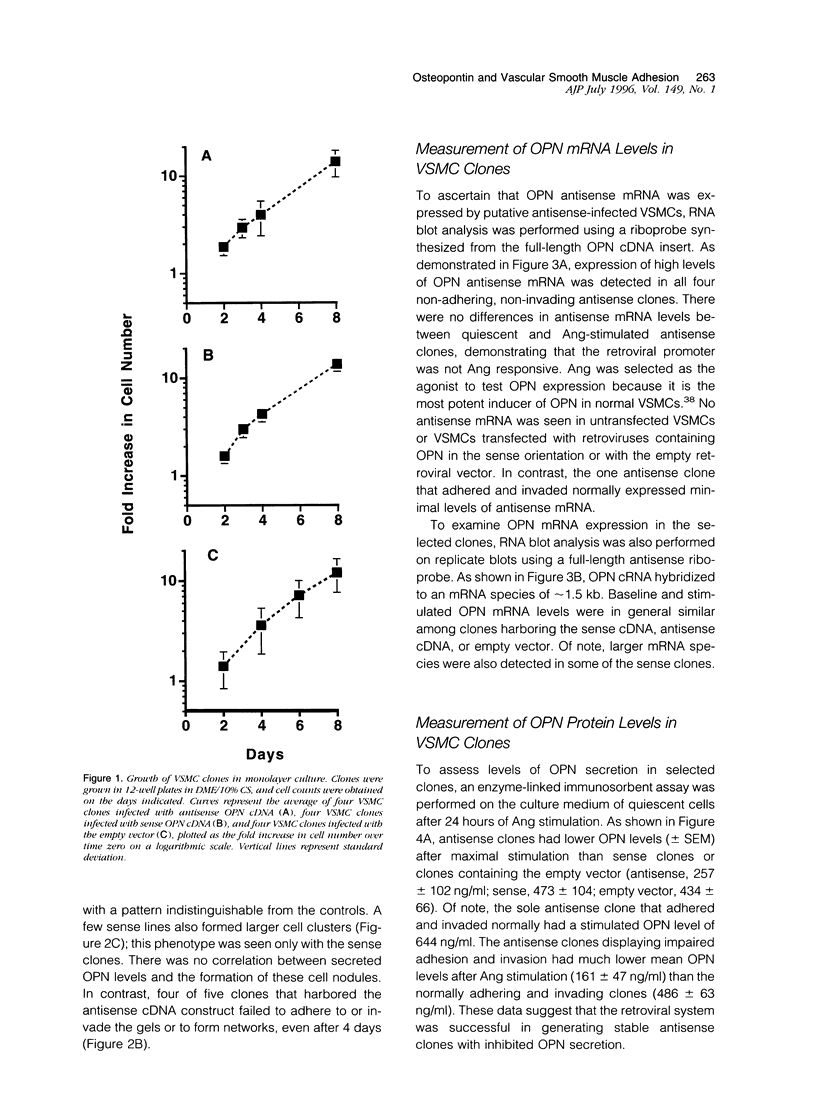
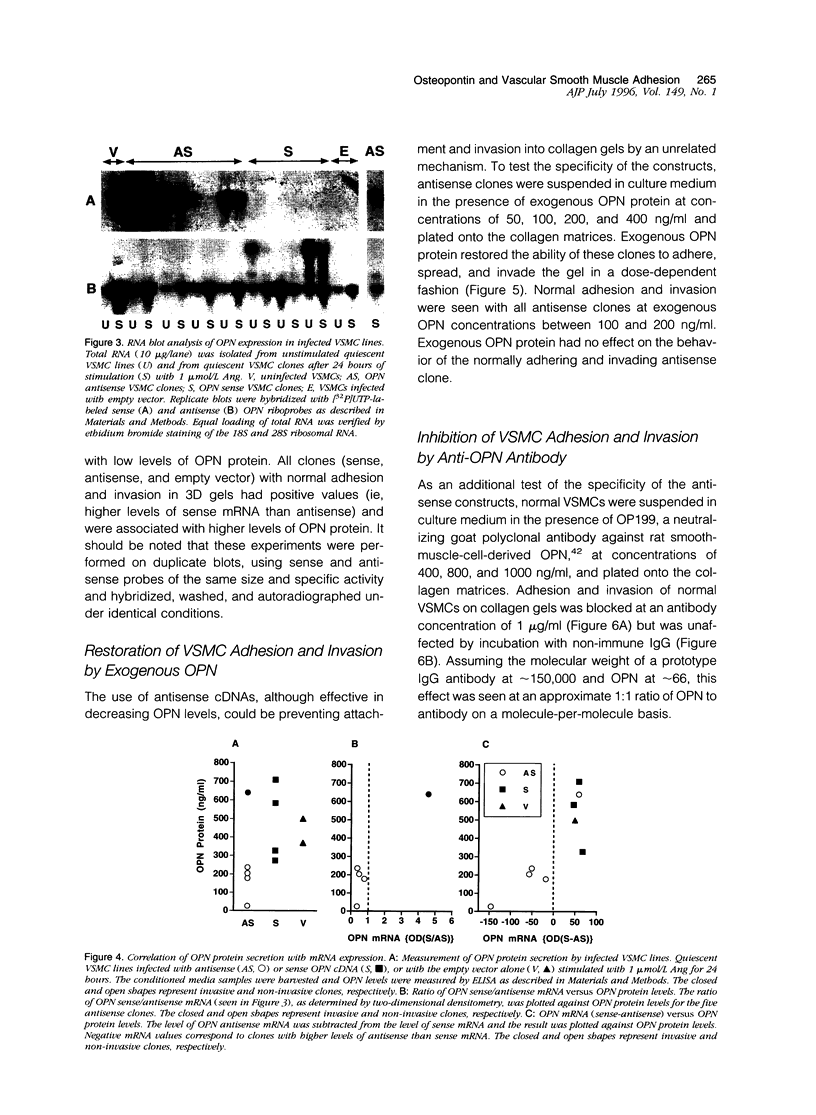
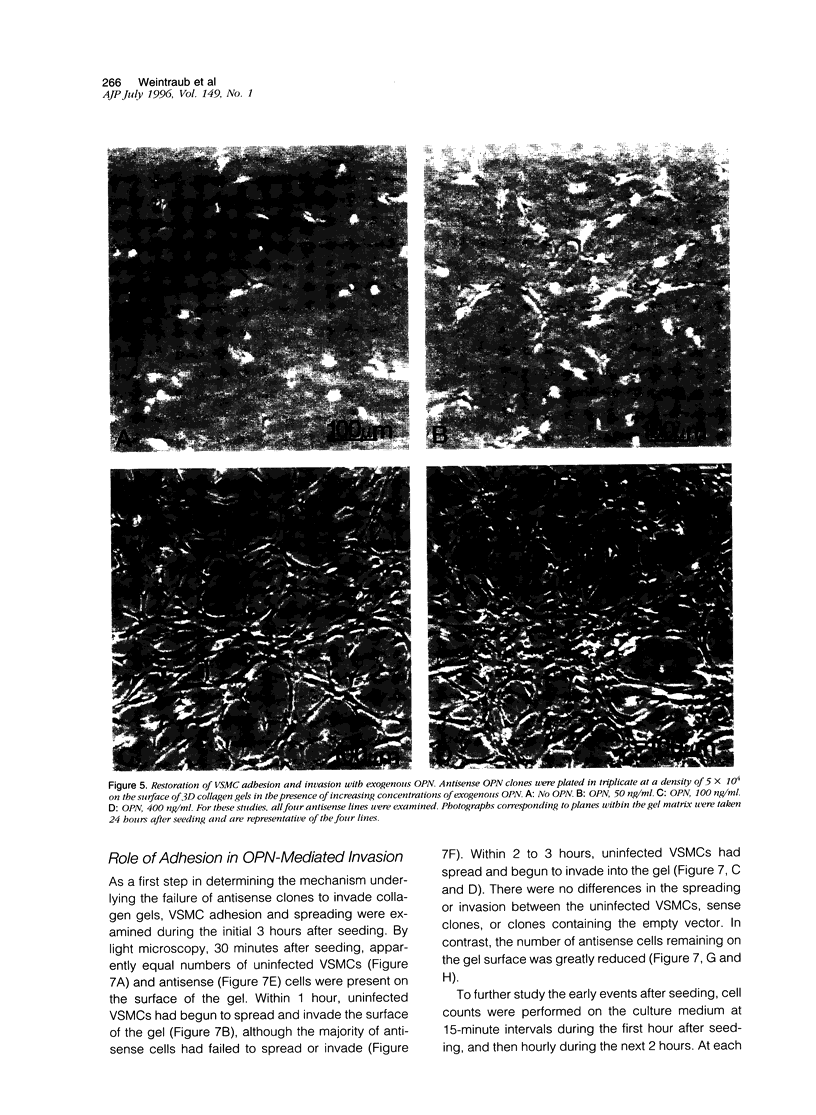
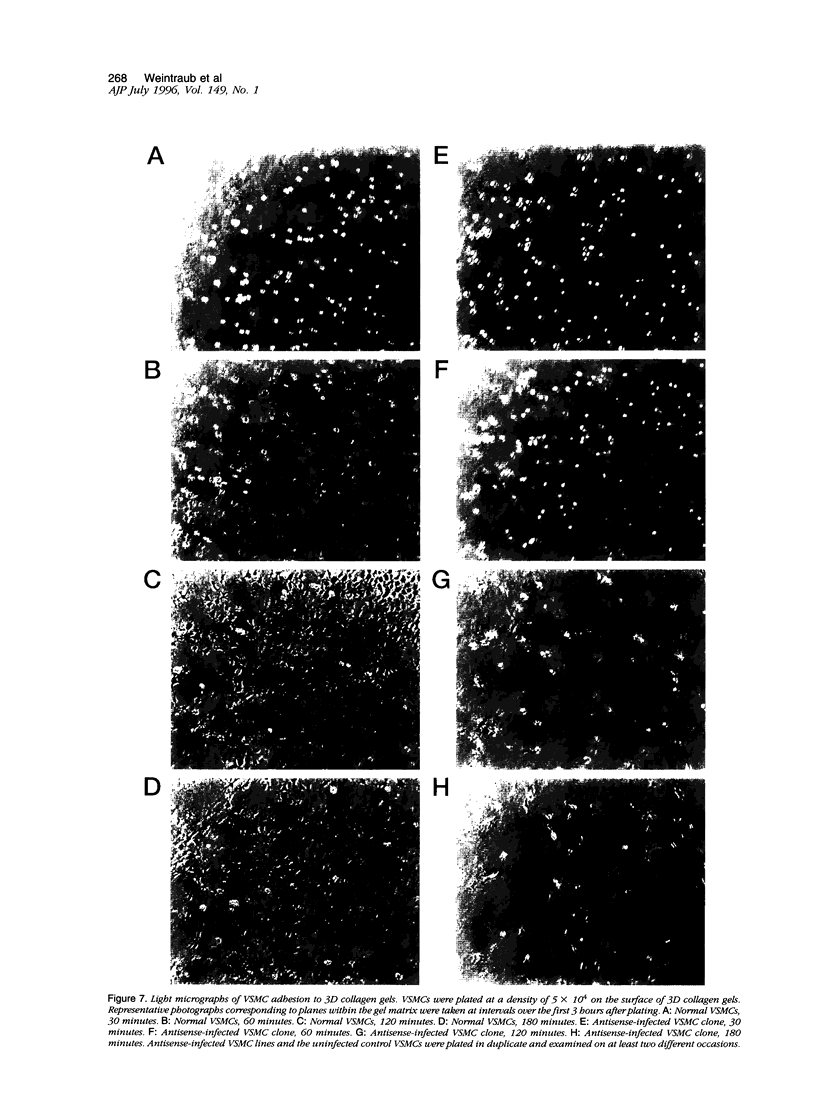
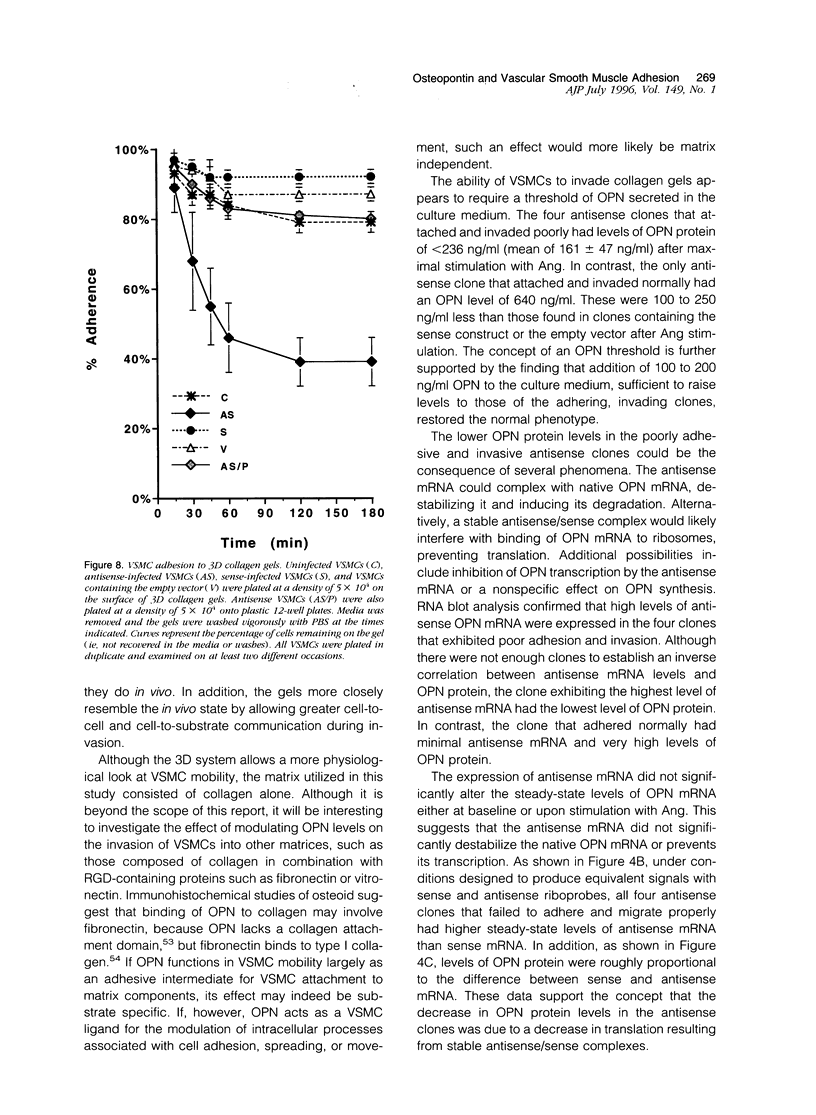
Osteopontin (OPN) is a secreted protein postulated to facilitate vascular smooth muscle cell (VSMC) adhesion and migration. Rat aortic VSMC lines were isolated after infection with recombinant retroviruses harboring OPN sense and antisense constructs. All lines grew normally in monolayer culture. On three-dimensional collagen gels, normal VSMCs and lines containing sense constructs (n=15) or empty vector (n=10) attached to gel and invaded the matrix. Four of five antisense clones did not adhere or invade. Antisense clones had lower OPN levels after stimulation with angiotensin II than sense clones or clones containing the empty vector (antisense, 257+/-102 ng/ml; sense, 473+/-104; vector, 434+/-66). Non-adhering antisense clones had lower mean OPN levels after angiotensin II stimulation (161+/-47 ng/ml) than sense or antisense lines with normal adhesion (486+/-63 ng/ml). The ability to adhere correlated with OPN levels >250 ng/ml. Adhesion and invasion were fully restored with addition of 100 to 200 ng/ml of exogenous OPN and were inhibited in normal VSMCs by incubation with 1 microgram/ml anti-OPN antibody. The autocrine secretion of OPN appears to play an important role in VSMC adhesion, spreading, and invasion.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernanke D. H., Markwald R. R. Effects of two glycosaminoglycans on seeding of cardiac cushion tissue cells into a collagen-lattice culture system. Anat Rec. 1984 Sep;210(1):25–31. doi: 10.1002/ar.1092100105. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Turley E., Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991 Feb;143(2):235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Brand S. J., Stone D. Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. J Clin Invest. 1988 Sep;82(3):1059–1066. doi: 10.1172/JCI113662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Cacace A. M., Guadagno S. N., Krauss R. S., Fabbro D., Weinstein I. B. The epsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene. 1993 Aug;8(8):2095–2104. [PubMed] [Google Scholar]
- Califf R. M., Fortin D. F., Frid D. J., Harlan W. R., 3rd, Ohman E. M., Bengtson J. R., Nelson C. L., Tcheng J. E., Mark D. B., Stack R. S. Restenosis after coronary angioplasty: an overview. J Am Coll Cardiol. 1991 May;17(6 Suppl B):2B–13B. doi: 10.1016/0735-1097(91)90933-z. [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Hota C., Prince C. W. Adhesion of metastatic, ras-transformed NIH 3T3 cells to osteopontin, fibronectin, and laminin. Cancer Res. 1993 Feb 1;53(3):701–706. [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Fingerle J., Reidy M. A. Regulation of smooth muscle cell growth in injured artery. J Cardiovasc Pharmacol. 1989;14 (Suppl 6):S12–S15. [PubMed] [Google Scholar]
- Delvos U., Gajdusek C., Sage H., Harker L. A., Schwartz S. M. Interactions of vascular wall cells with collagen gels. Lab Invest. 1982 Jan;46(1):61–72. [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Gadeau A. P., Campan M., Millet D., Candresse T., Desgranges C. Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb. 1993 Jan;13(1):120–125. doi: 10.1161/01.atv.13.1.120. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Muszynski M., Sukhova G. K., Simon-Morrissey E., Unemori E. N., Lark M. W., Amento E., Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994 Jul;75(1):181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- Gardner H. A., Berse B., Senger D. R. Specific reduction in osteopontin synthesis by antisense RNA inhibits the tumorigenicity of transformed Rat1 fibroblasts. Oncogene. 1994 Aug;9(8):2321–2326. [PubMed] [Google Scholar]
- Garratt K. N., Edwards W. D., Kaufmann U. P., Vlietstra R. E., Holmes D. R., Jr Differential histopathology of primary atherosclerotic and restenotic lesions in coronary arteries and saphenous vein bypass grafts: analysis of tissue obtained from 73 patients by directional atherectomy. J Am Coll Cardiol. 1991 Feb;17(2):442–448. doi: 10.1016/s0735-1097(10)80113-5. [DOI] [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C. M., Pichler R., Lombardi D., Denhardt D. T., Alpers C. E., Schwartz S. M., Johnson R. J. Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int. 1994 Feb;45(2):515–524. doi: 10.1038/ki.1994.67. [DOI] [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Green R. S., Lieb M. E., Weintraub A. S., Gacheru S. N., Rosenfield C. L., Shah S., Kagan H. M., Taubman M. B. Identification of lysyl oxidase and other platelet-derived growth factor-inducible genes in vascular smooth muscle cells by differential screening. Lab Invest. 1995 Oct;73(4):476–482. [PubMed] [Google Scholar]
- Hedin U., Holm J., Hansson G. K. Induction of tenascin in rat arterial injury. Relationship to altered smooth muscle cell phenotype. Am J Pathol. 1991 Sep;139(3):649–656. [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Rabinovitch M. 67-kD elastin-binding protein is a protective "companion" of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol. 1994 Jul;126(2):563–574. doi: 10.1083/jcb.126.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. M., Lopez C. A., Heck D. E., Gardner C. R., Laskin D. L., Laskin J. D., Denhardt D. T. Osteopontin inhibits induction of nitric oxide synthase gene expression by inflammatory mediators in mouse kidney epithelial cells. J Biol Chem. 1994 Jan 7;269(1):711–715. [PubMed] [Google Scholar]
- Irving J. A., Lala P. K. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995 Apr;217(2):419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J., Fietzek P. P., Woolley D. E. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem. 1978 Aug 25;253(16):5642–5646. [PubMed] [Google Scholar]
- Liaw L., Almeida M., Hart C. E., Schwartz S. M., Giachelli C. M. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994 Feb;74(2):214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Bell L., Marx M., Merwin J. R., Basson C., Prinz C. Effects of soluble factors and extracellular matrix components on vascular cell behavior in vitro and in vivo: models of de-endothelialization and repair. J Cell Biochem. 1991 Feb;45(2):123–130. doi: 10.1002/jcb.240450202. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Marx M. Matrix composition, organization and soluble factors: modulators of microvascular cell differentiation in vitro. Kidney Int. 1992 Mar;41(3):560–565. doi: 10.1038/ki.1992.82. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W. Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. Tex Heart Inst J. 1994;21(1):78–85. [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniccia R., Colucci S., Grano M., Serra M., Zallone A. Z., Teti A. Immediate cell signal by bone-related peptides in human osteoclast-like cells. Am J Physiol. 1993 Nov;265(5 Pt 1):C1289–C1297. doi: 10.1152/ajpcell.1993.265.5.C1289. [DOI] [PubMed] [Google Scholar]
- Pauly R. R., Passaniti A., Bilato C., Monticone R., Cheng L., Papadopoulos N., Gluzband Y. A., Smith L., Weinstein C., Lakatta E. G. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994 Jul;75(1):41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
- Pickering J. G., Weir L., Jekanowski J., Kearney M. A., Isner J. M. Proliferative activity in peripheral and coronary atherosclerotic plaque among patients undergoing percutaneous revascularization. J Clin Invest. 1993 Apr;91(4):1469–1480. doi: 10.1172/JCI116352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Raugi G. J., Mullen J. S., Bark D. H., Okada T., Mayberg M. R. Thrombospondin deposition in rat carotid artery injury. Am J Pathol. 1990 Jul;137(1):179–185. [PMC free article] [PubMed] [Google Scholar]
- RayChaudhury A., Frazier W. A., D'Amore P. A. Comparison of normal and tumorigenic endothelial cells: differences in thrombospondin production and responses to transforming growth factor-beta. J Cell Sci. 1994 Jan;107(Pt 1):39–46. doi: 10.1242/jcs.107.1.39. [DOI] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989 Sep-Oct;9(5):1291–1299. [PubMed] [Google Scholar]
- Simchen G., Hugerat Y. What determines whether chromosomes segregate reductionally or equationally in meiosis? Bioessays. 1993 Jan;15(1):1–8. doi: 10.1002/bies.950150102. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Strauss B. H., Chisholm R. J., Keeley F. W., Gotlieb A. I., Logan R. A., Armstrong P. W. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994 Oct;75(4):650–658. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Marmur J. D., Rosenfield C. L., Guha A., Nichtberger S., Nemerson Y. Agonist-mediated tissue factor expression in cultured vascular smooth muscle cells. Role of Ca2+ mobilization and protein kinase C activation. J Clin Invest. 1993 Feb;91(2):547–552. doi: 10.1172/JCI116234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley E. A., Erickson C. A., Tucker R. P. The retention and ultrastructural appearances of various extracellular matrix molecules incorporated into three-dimensional hydrated collagen lattices. Dev Biol. 1985 Jun;109(2):347–369. doi: 10.1016/0012-1606(85)90461-0. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Parhar R. S., Guo X., Lala P. K., Denhardt D. T. Regulated temporal and spatial expression of the calcium-binding proteins calcyclin and OPN (osteopontin) in mouse tissues during pregnancy. Mol Reprod Dev. 1992 Aug;32(4):315–323. doi: 10.1002/mrd.1080320403. [DOI] [PubMed] [Google Scholar]
- Wax S. D., Rosenfield C. L., Taubman M. B. Identification of a novel growth factor-responsive gene in vascular smooth muscle cells. J Biol Chem. 1994 Apr 29;269(17):13041–13047. [PubMed] [Google Scholar]
- Wren F. E., Schor A. M., Schor S. L., Grant M. E. Modulation of smooth muscle cell behaviour by platelet-derived factors and the extracellular matrix. J Cell Physiol. 1986 May;127(2):297–302. doi: 10.1002/jcp.1041270217. [DOI] [PubMed] [Google Scholar]
- Yanagi H., Sasaguri Y., Sugama K., Morimatsu M., Nagase H. Production of tissue collagenase (matrix metalloproteinase 1) by human aortic smooth muscle cells in response to platelet-derived growth factor. Atherosclerosis. 1991 Dec;91(3):207–216. doi: 10.1016/0021-9150(91)90168-3. [DOI] [PubMed] [Google Scholar]