Abstract
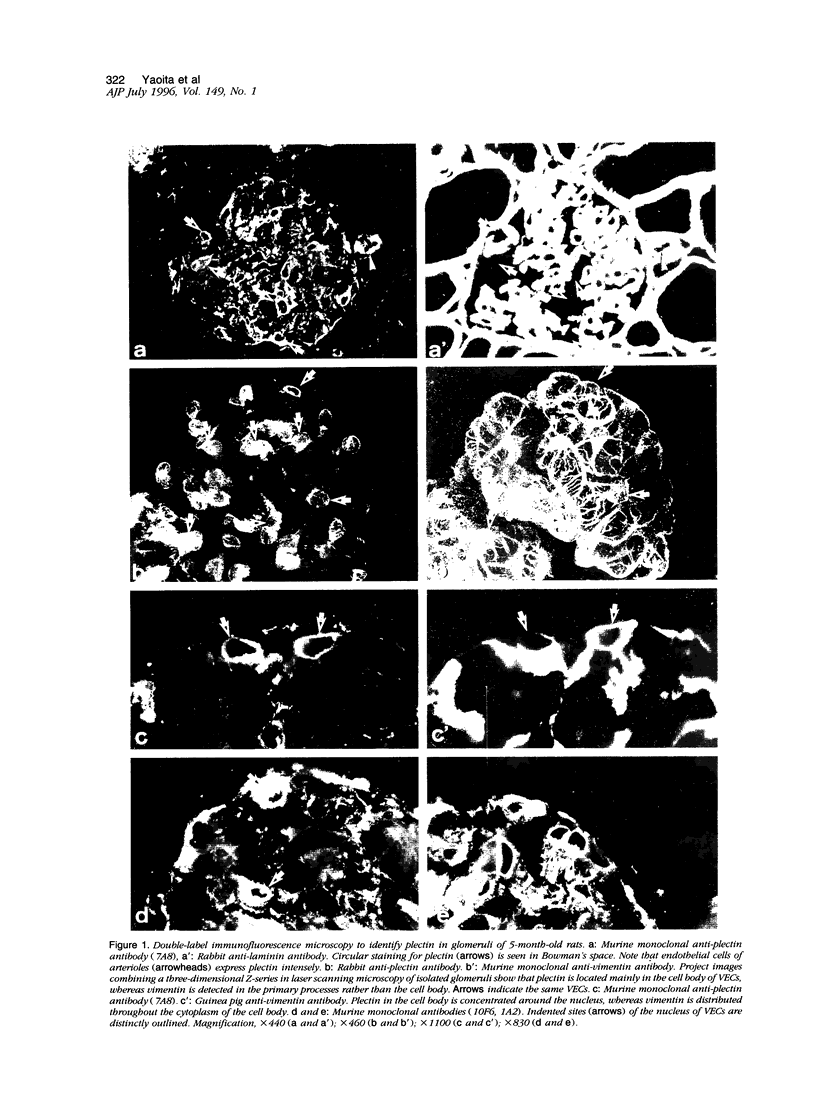
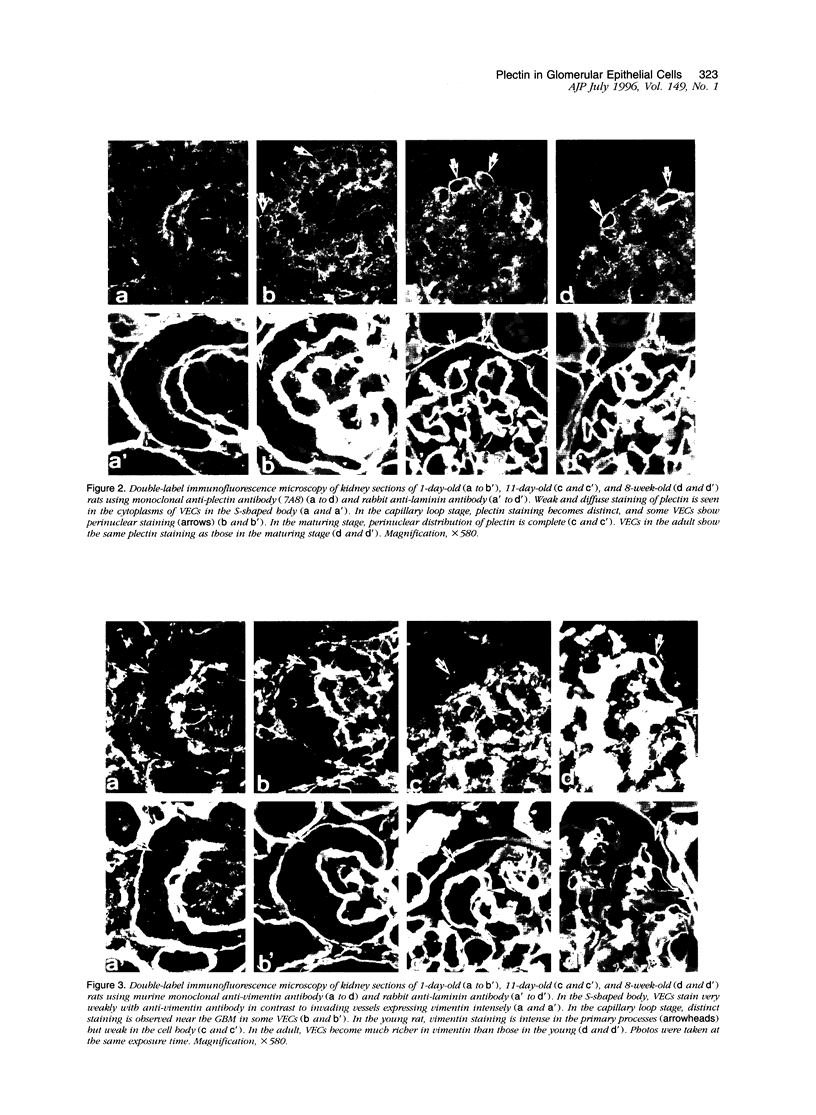
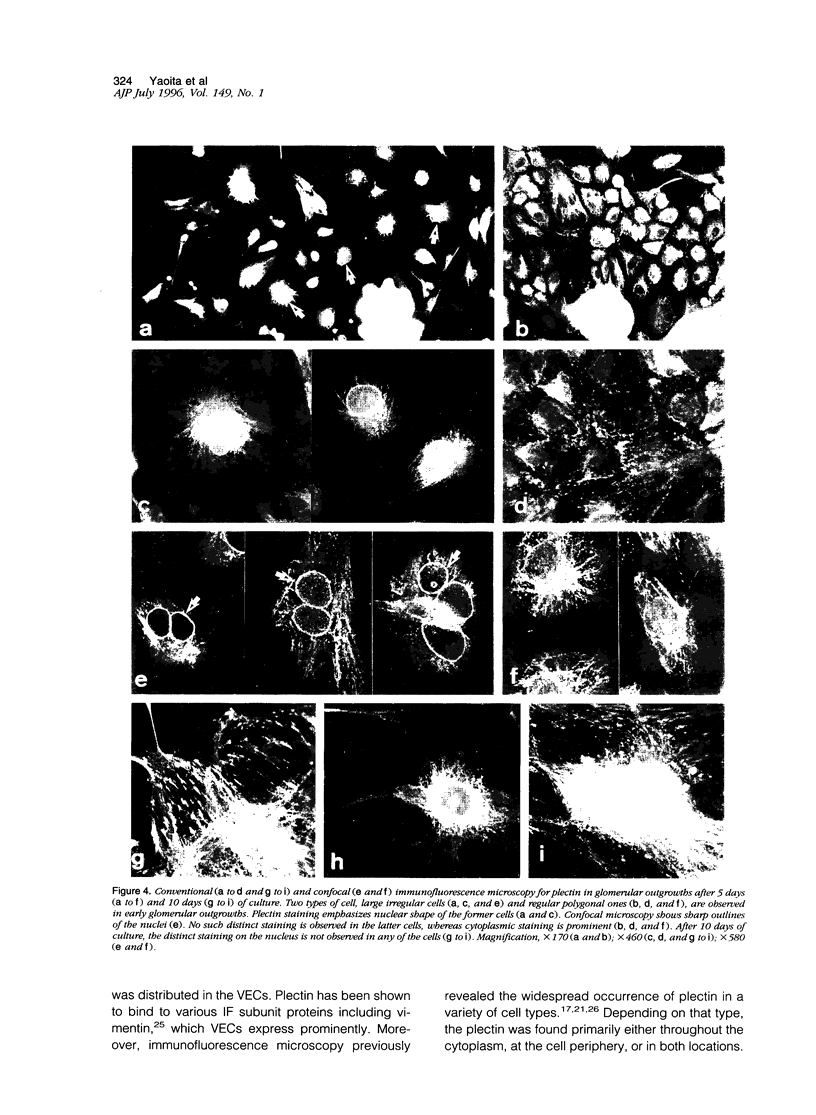
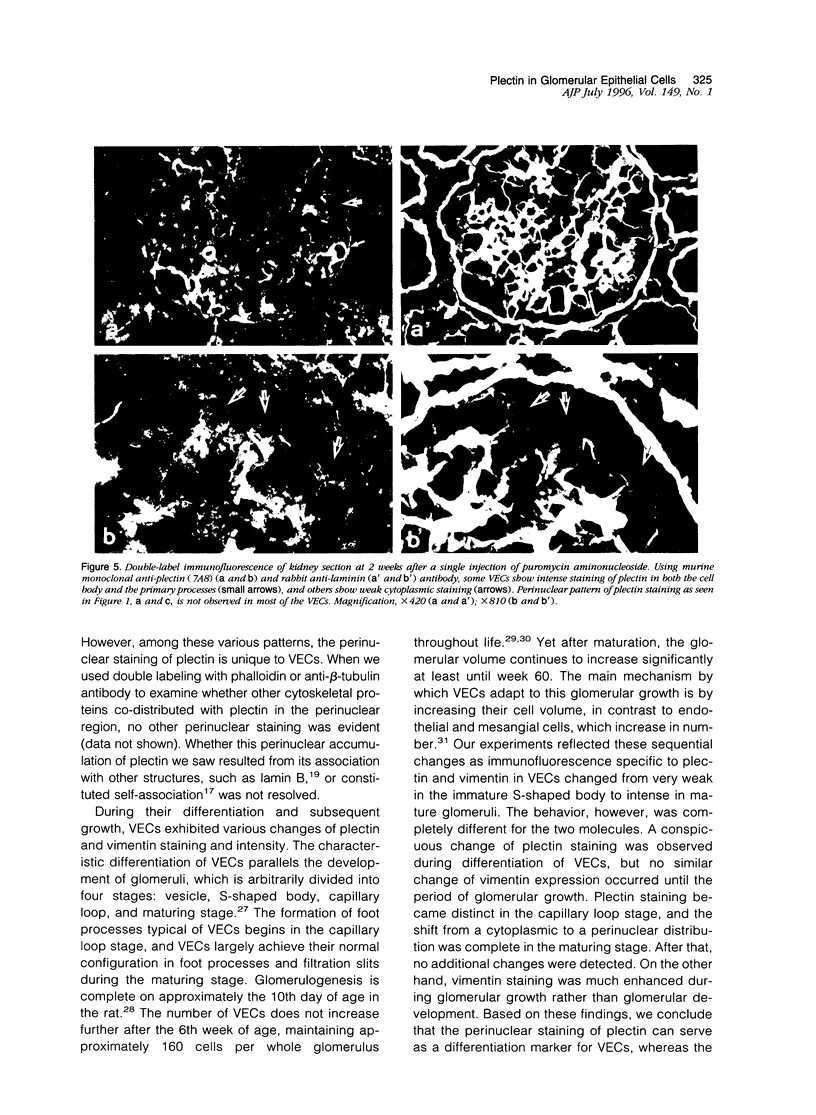
Plectin is an intermediate-filament-associated protein identified over a wide range of tissue and cell types. The distribution of this protein in glomerular visceral epithelial cells (VECs) during the differentiation and growth of rat kidneys was studied in comparison with that of vimentin. By immunofluorescence microscopy, preferential localization of these two cytomatrix elements was different, although both were observed in the cell body and primary processes of VECs. Strong staining of plectin was always found in the perinuclear region of the VEC body in kidneys of young and adult rat, but vimentin stained distinctly only in the primary processes of young rats yet in both cell bodies and primary processes of the adults. This perinuclear staining was unique to VECs, that is, was absent from other cells. In the neonatal kidney, plectin staining during differentiation of VECs changed from weak and diffuse throughout the cytoplasm in the S-shaped body to prominently perinuclear in the maturing stage. However, after the differentiation of VECs, the staining intensity of plectin did not change further. In contrast, that of vimentin increased conspicuously in parallel with the growth of VECs rather than at differentiation. After a long period of culture and during aminonucleoside nephrosis, situations when VECs lose differentiated phenotypes, most of the cells had no perinuclear plectin. These findings indicate that the perinuclear distribution of plectin may play an important role in the differentiation of VECs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Herin P. Development of glomerular perfusion rate and nephron filtration rate in rats 17-60 days old. Am J Physiol. 1975 May;228(5):1319–1325. doi: 10.1152/ajplegacy.1975.228.5.1319. [DOI] [PubMed] [Google Scholar]
- Bachmann S., Kriz W., Kuhn C., Franke W. W. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77(3):365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- Drenckhahn D., Franke R. P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988 Nov;59(5):673–682. [PubMed] [Google Scholar]
- Errante L. D., Wiche G., Shaw G. Distribution of plectin, an intermediate filament-associated protein, in the adult rat central nervous system. J Neurosci Res. 1994 Mar 1;37(4):515–528. doi: 10.1002/jnr.490370411. [DOI] [PubMed] [Google Scholar]
- Foisner R., Feldman B., Sander L., Wiche G. Monoclonal antibody mapping of structural and functional plectin epitopes. J Cell Biol. 1991 Feb;112(3):397–405. doi: 10.1083/jcb.112.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Leichtfried F. E., Herrmann H., Small J. V., Lawson D., Wiche G. Cytoskeleton-associated plectin: in situ localization, in vitro reconstitution, and binding to immobilized intermediate filament proteins. J Cell Biol. 1988 Mar;106(3):723–733. doi: 10.1083/jcb.106.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Traub P., Wiche G. Protein kinase A- and protein kinase C-regulated interaction of plectin with lamin B and vimentin. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3812–3816. doi: 10.1073/pnas.88.9.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Wiche G. Intermediate filament-associated proteins. Curr Opin Cell Biol. 1991 Feb;3(1):75–81. doi: 10.1016/0955-0674(91)90168-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Fries J. W., Sandstrom D. J., Meyer T. W., Rennke H. G. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab Invest. 1989 Feb;60(2):205–218. [PubMed] [Google Scholar]
- Herrmann H., Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987 Jan 25;262(3):1320–1325. [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Lehto V. P., Lehtonen E., Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest. 1984 May;50(5):552–559. [PubMed] [Google Scholar]
- Holthöfer H., Sainio K., Miettinen A. Rat glomerular cells do not express podocytic markers when cultured in vitro. Lab Invest. 1991 Nov;65(5):548–557. [PubMed] [Google Scholar]
- Janmey P. A., Euteneuer U., Traub P., Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991 Apr;113(1):155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara I., Yatoita E., Kawasaki K., Yamamoto T. Limitations of podocyte adaptation for glomerular injury in puromycin aminonucleoside nephrosis. Pathol Int. 1995 Sep;45(9):625–634. doi: 10.1111/j.1440-1827.1995.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Moll R., Hage C., Thoenes W. Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest. 1991 Jul;65(1):74–86. [PubMed] [Google Scholar]
- Nagata M., Kriz W. Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int. 1992 Jul;42(1):148–160. doi: 10.1038/ki.1992.272. [DOI] [PubMed] [Google Scholar]
- Nagata M., Yamaguchi Y., Ito K. Loss of mitotic activity and the expression of vimentin in glomerular epithelial cells of developing human kidneys. Anat Embryol (Berl) 1993 Mar;187(3):275–279. doi: 10.1007/BF00195765. [DOI] [PubMed] [Google Scholar]
- Nörgaard J. O. Retraction of epithelial foot processes during culture of isolated glomeruli. Lab Invest. 1978 Mar;38(3):320–329. [PubMed] [Google Scholar]
- Nørgaard J. O. Rat glomerular epithelial cells in culture. Parietal or visceral epithelial origin? Lab Invest. 1987 Sep;57(3):277–290. [PubMed] [Google Scholar]
- Okasora T., Nagase M., Kawachi H., Matsui K., Orikasa M., Morioka T., Yamazaki I., Oite T., Shimizu F. Altered localization of antigen recognized by proteinuria-inducing monoclonal antibody in experimental nephrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(1):41–46. doi: 10.1007/BF02899526. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Anversa P., Melissari M., Loud A. V. Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int. 1980 Apr;17(4):438–454. doi: 10.1038/ki.1980.52. [DOI] [PubMed] [Google Scholar]
- Pabst R., Sterzel R. B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983 Nov;24(5):626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- Pricam C., Humbert F., Perrelet A., Amherdt M., Orci L. Intercellular junctions in podocytes of the nephrotic glomerulus as seen with freeze-fracture. Lab Invest. 1975 Sep;33(3):209–218. [PubMed] [Google Scholar]
- Rasch R., Nörgaard J. O. Renal enlargement: comparative autoradiographic studies of 3H-thymidine uptake in diabetic and uninephrectomized rats. Diabetologia. 1983 Sep;25(3):280–287. doi: 10.1007/BF00279944. [DOI] [PubMed] [Google Scholar]
- Reeves W., Caulfield J. P., Farquhar M. G. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978 Aug;39(2):90–100. [PubMed] [Google Scholar]
- Ryan G. B., Rodewald R., Karnovsky M. J. An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest. 1975 Nov;33(5):461–468. [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasmant D., Maurice M., Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec. 1984 Sep;210(1):17–24. doi: 10.1002/ar.1092100104. [DOI] [PubMed] [Google Scholar]
- Weinstein T., Cameron R., Katz A., Silverman M. Rat glomerular epithelial cells in culture express characteristics of parietal, not visceral, epithelium. J Am Soc Nephrol. 1992 Dec;3(6):1279–1287. doi: 10.1681/ASN.V361279. [DOI] [PubMed] [Google Scholar]
- Wiche G., Krepler R., Artlieb U., Pytela R., Denk H. Occurrence and immunolocalization of plectin in tissues. J Cell Biol. 1983 Sep;97(3):887–901. doi: 10.1083/jcb.97.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G. Plectin: general overview and appraisal of its potential role as a subunit protein of the cytomatrix. Crit Rev Biochem Mol Biol. 1989;24(1):41–67. doi: 10.3109/10409238909082551. [DOI] [PubMed] [Google Scholar]
- Yaoita E., Kawasaki K., Yamamoto T., Kihara I. Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol. 1990 Apr;136(4):899–908. [PMC free article] [PubMed] [Google Scholar]
- Yaoita E., Yamamoto T., Saito M., Kawasaki K., Kihara I. Desmin-positive epithelial cells outgrowing from rat encapsulated glomeruli. Eur J Cell Biol. 1991 Feb;54(1):140–149. [PubMed] [Google Scholar]
- Yaoita E., Yamamoto T., Takashima N., Kawasaki K., Kawachi H., Shimizu F., Kihara I. Visceral epithelial cells in rat glomerular cell culture. Eur J Cell Biol. 1995 Jun;67(2):136–144. [PubMed] [Google Scholar]