Abstract
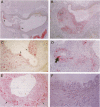
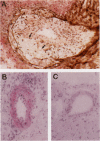
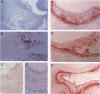
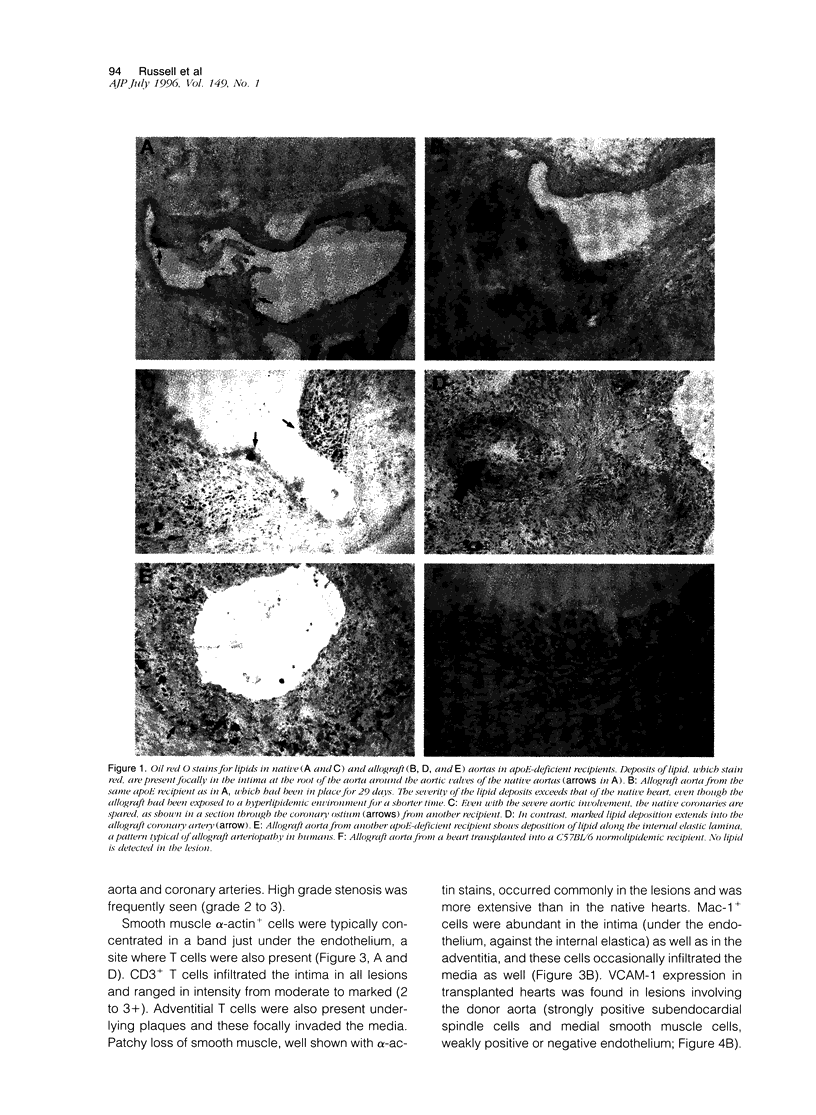
Arteriopathy, sometimes termed accelerated atherosclerosis, often impairs transplants. We employed apolipoprotein-E-deficient, hypercholesterolemic mice to determine how the hyperlipidemic environment affected transplanted hearts. Strain 129 hearts transplanted to C57BL/6 normal or C57BL/6 apolipoprotein-E-deficient recipients were evaluated by immunochemical and histological techniques. Analyses were possible both of differences in the coronary lesions that developed in a normolipidemic as compared with a hyperlipidemic environment and of the coronary atherosclerotic process in transplanted hearts compared with native hearts in the same hyperlipidemic environment. Aortas and coronary arteries of transplanted aortas in both recipient groups developed florid intimal thickening by 4 to 10 weeks, with marked lipid deposition, foamy macrophages, and infiltration of smooth muscle alpha-actin-positive cells in apolipoprotein-E-deficient mice. Lipid was layered against the internal elastic lamina as in human transplants. VCAM-1 was increased in various sites in both groups. Allotransplants to apolipoprotein-E-deficient recipients had more severe aortic and coronary lesions with characteristic T cell infiltration than native hearts. In this sense, transplants suffered from accelerated atherosclerosis. The character of coronary vascular changes in transplanted hearts was distinctly affected by their lipid environment, but their severity, in terms of luminal encroachment, was not markedly different.
Full text
PDF



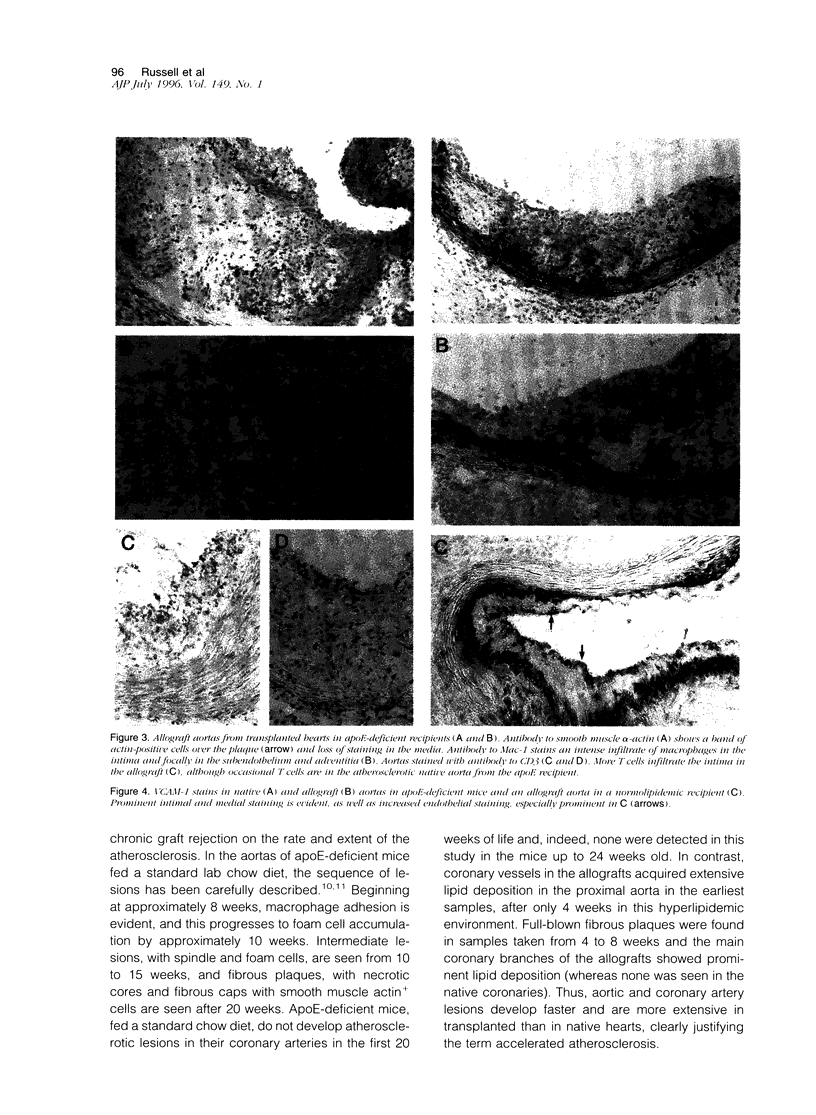



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Karnovsky M. J. Hypercholesterolemia does not exacerbate arterial intimal thickening in chronically rejecting rat cardiac allografts. Transplant Proc. 1989 Feb;21(1 Pt 1):437–439. [PubMed] [Google Scholar]
- Alonso D. R., Starek P. K., Minick C. R. Studies on the pathogenesis of atheroarteriosclerosis induced in rabbit cardiac allografts by the synergy of graft rejection and hypercholesterolemia. Am J Pathol. 1977 May;87(2):415–442. [PMC free article] [PubMed] [Google Scholar]
- Ardehali A., Laks H., Drinkwater D. C., Ziv E., Drake T. A. Vascular cell adhesion molecule-1 is induced on vascular endothelia and medial smooth muscle cells in experimental cardiac allograft vasculopathy. Circulation. 1995 Aug 1;92(3):450–456. doi: 10.1161/01.cir.92.3.450. [DOI] [PubMed] [Google Scholar]
- Bacchi C. E., Marsh C. L., Perkins J. D., Carithers R. L., Jr, McVicar J. P., Hudkins K. L., Benjamin C. D., Harlan J. M., Lobb R., Alpers C. E. Expression of vascular cell adhesion molecule (VCAM-1) in liver and pancreas allograft rejection. Am J Pathol. 1993 Feb;142(2):579–591. [PMC free article] [PubMed] [Google Scholar]
- Breslow J. L. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B. The pathogenesis of vascular rejection. Transplant Proc. 1991 Aug;23(4):2052–2055. [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Ferran C., Peuchmaur M., Desruennes M., Ghoussoub J. J., Cabrol A., Brousse N., Cabrol C., Bach J. F., Chatenoud L. Implications of de novo ELAM-1 and VCAM-1 expression in human cardiac allograft rejection. Transplantation. 1993 Mar;55(3):605–609. doi: 10.1097/00007890-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Frostegård J., Wu R., Haegerstrand A., Patarroyo M., Lefvert A. K., Nilsson J. Mononuclear leukocytes exposed to oxidized low density lipoprotein secrete a factor that stimulates endothelial cells to express adhesion molecules. Atherosclerosis. 1993 Nov;103(2):213–219. doi: 10.1016/0021-9150(93)90264-u. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992 Jan 23;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Gibbs P., Berkley L. M., Bolton E. M., Briggs J. D., Bradley J. A. Adhesion molecule expression (ICAM-1, VCAM-1, E-selectin and PECAM) in human kidney allografts. Transpl Immunol. 1993;1(2):109–113. doi: 10.1016/0966-3274(93)90003-q. [DOI] [PubMed] [Google Scholar]
- Isobe M., Suzuki J., Yagita H., Okumura K., Yamazaki S., Nagai R., Yazaki Y., Sekiguchi M. Immunosuppression to cardiac allografts and soluble antigens by anti-vascular cellular adhesion molecule-1 and anti-very late antigen-4 monoclonal antibodies. J Immunol. 1994 Dec 15;153(12):5810–5818. [PubMed] [Google Scholar]
- Khan B. V., Parthasarathy S. S., Alexander R. W., Medford R. M. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995 Mar;95(3):1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993 Dec;143(6):1551–1559. [PMC free article] [PubMed] [Google Scholar]
- Lurie K. G., Billingham M. E., Jamieson S. W., Harrison D. C., Reitz B. A. Pathogenesis and prevention of graft arteriosclerosis in an experimental heart transplant model. Transplantation. 1981 Jan;31(1):41–47. doi: 10.1097/00007890-198101000-00010. [DOI] [PubMed] [Google Scholar]
- McManus B. M., Horley K. J., Wilson J. E., Malcom G. T., Kendall T. J., Miles R. R., Winters G. L., Costanzo M. R., Miller L. L., Radio S. J. Prominence of coronary arterial wall lipids in human heart allografts. Implications for pathogenesis of allograft arteriopathy. Am J Pathol. 1995 Aug;147(2):293–308. [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Pelletier R. P., Morgan C. J., Sedmak D. D., Miyake K., Kincade P. W., Ferguson R. M., Orosz C. G. Analysis of inflammatory endothelial changes, including VCAM-1 expression, in murine cardiac grafts. Transplantation. 1993 Feb;55(2):315–320. doi: 10.1097/00007890-199302000-00017. [DOI] [PubMed] [Google Scholar]
- Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Winn H. J., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. I. Time course and immunogenetic and immunopathological considerations. Am J Pathol. 1994 Feb;144(2):260–274. [PMC free article] [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Winn H. J., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994 May 15;152(10):5135–5141. [PubMed] [Google Scholar]
- Savage C. O., Hughes C. C., McIntyre B. W., Picard J. K., Pober J. S. Human CD4+ T cells proliferate to HLA-DR+ allogeneic vascular endothelium. Identification of accessory interactions. Transplantation. 1993 Jul;56(1):128–134. doi: 10.1097/00007890-199307000-00024. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Libby P. Interaction of the allogeneic state and hypercholesterolemia in arterial lesion formation in experimental cardiac allografts. Arterioscler Thromb. 1994 May;14(5):734–745. doi: 10.1161/01.atv.14.5.734. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Swanson S. J., Cybulsky M. I., Schoen F. J., Libby P. Endothelial and smooth muscle cells express leukocyte adhesion molecules heterogeneously during acute rejection of rabbit cardiac allografts. Am J Pathol. 1994 May;144(5):938–951. [PMC free article] [PubMed] [Google Scholar]
- Turunen J. P., Paavonen T., Majuri M. L., Tiisala S., Mattila P., Mennander A., Gahmberg C. G., Häyry P., Tamatani T., Miyasaka M. Sialyl Lewis(x)- and L-selectin-dependent site-specific lymphocyte extravasation into renal transplants during acute rejection. Eur J Immunol. 1994 May;24(5):1130–1136. doi: 10.1002/eji.1830240518. [DOI] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]