Abstract
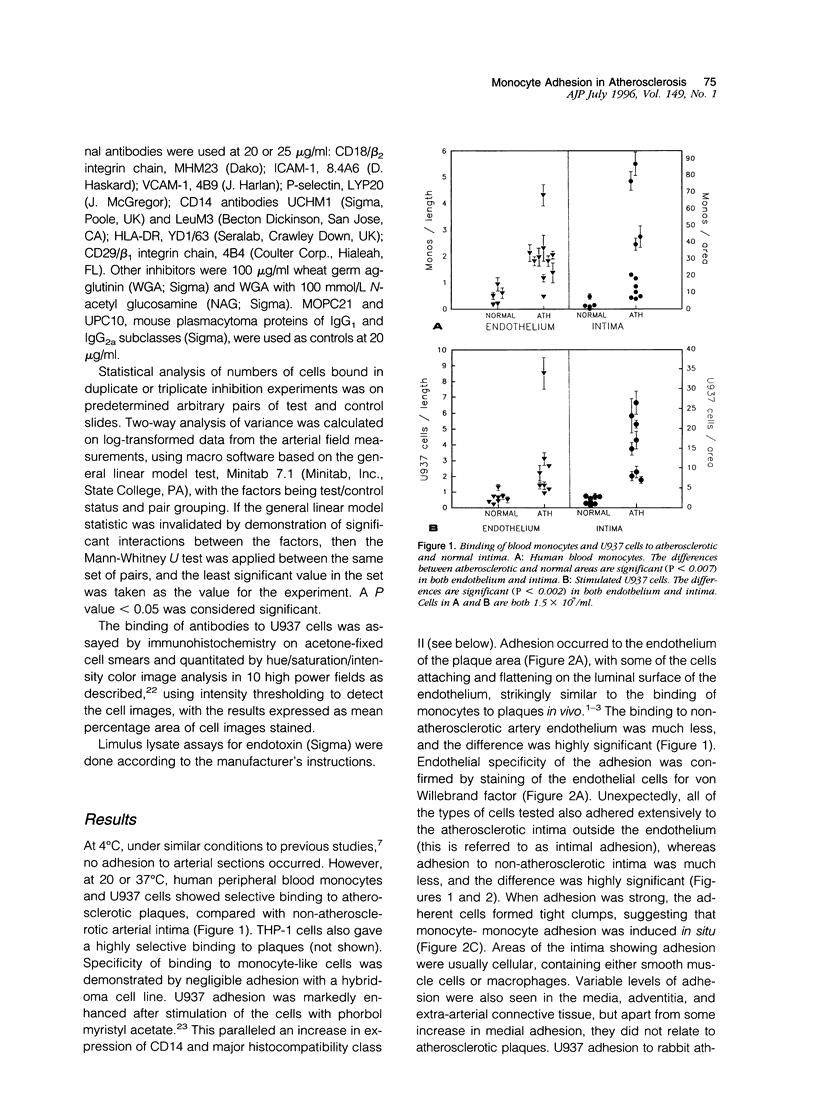
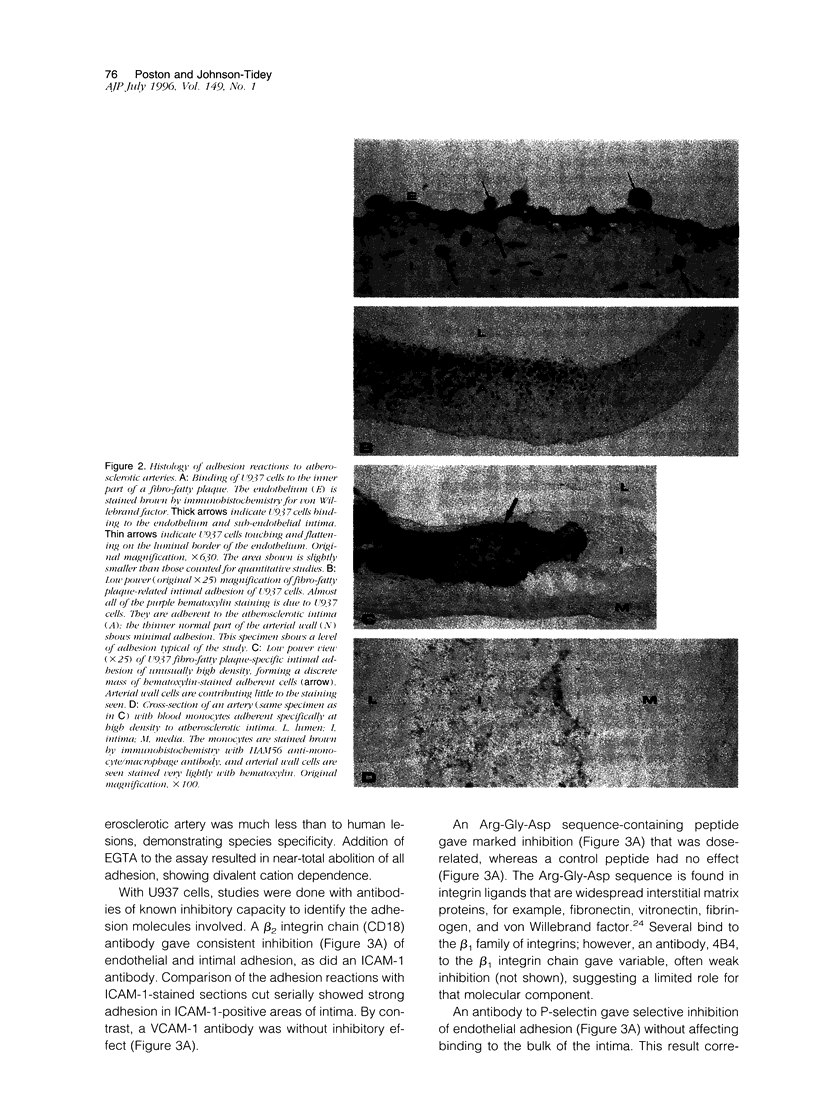
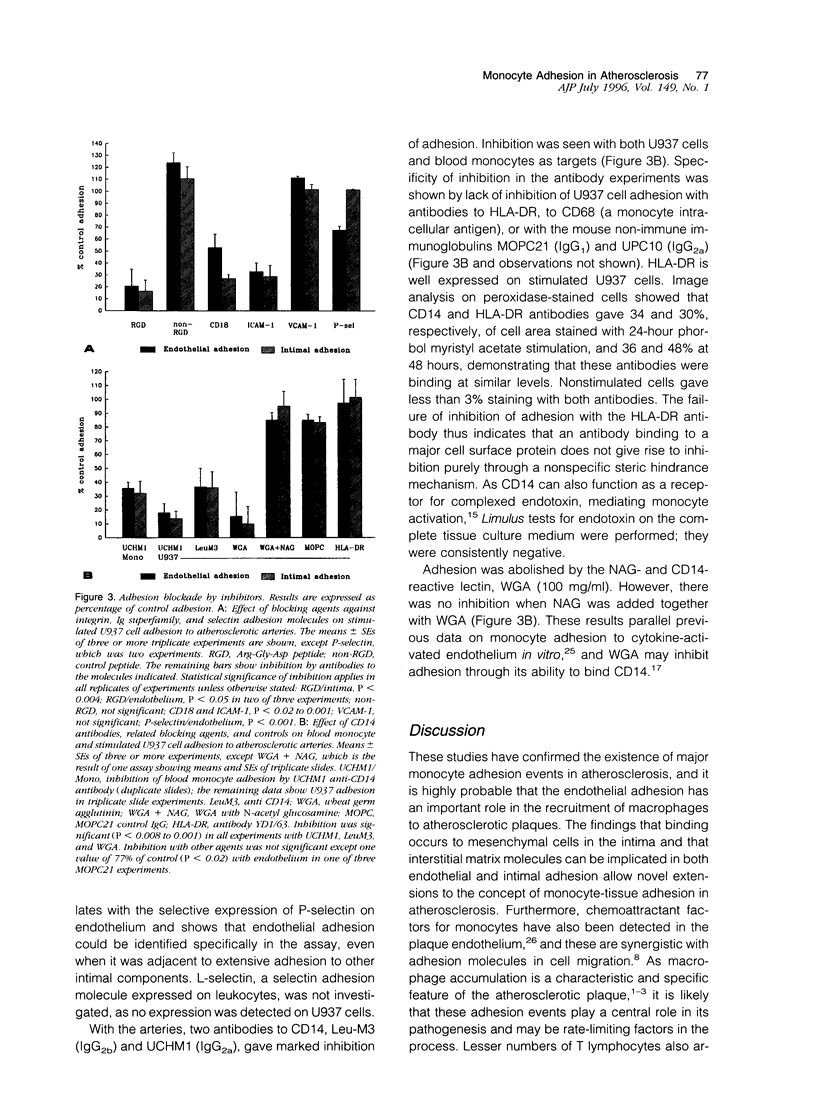
Blood-derived macrophages in the arterial intima are a characteristic feature of active atherosclerotic plaques. Adherent monocytes on the luminal surface and increased adhesion molecules on the endothelium have suggested that specific molecular mechanisms are involved in monocyte/macrophage traffic into the arterial wall. Adhesion of human monocytes and related cell lines was therefore studied in vitro to histological sections of human plaques. At 37 degrees C, these cells bound selectively to the plaques. Binding to the endothelium occurred and was also present extensively in the diseased intima. Inhibition studies showed that the endothelial and general intimal binding had largely similar molecular properties. Strong inhibition was produced by antibodies to the monocyte-specific adhesion molecule CD14, to beta2 integrins, and to ICAM-1. Likewise, a peptide containing the Arg-Gly-Asp sequence was strongly inhibitory, suggesting that binding of leukocyte integrins to arterial extracellular matrix was synergistic with cell-cell interactions. A P-selectin antibody was exceptional in giving selective inhibition of endothelial adhesion, which correlates with the specific endothelial localization of this adhesion molecule. These results show that monocytes adhere to atherosclerotic plaques through the focal activation of multiple arterial wall adhesion molecules, confirming the adhesion hypothesis. A positive feedback theory for the pathogenesis of atherosclerosis can be suggested, based on the ability of macrophages in the wall to activate the endothelium, induce adhesion molecules, and facilitate additional monocyte entry. The adhesion assay provides a means for the identification of adhesion inhibitors with therapeutic potential.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beekhuizen H., Blokland I., Corsèl-van Tilburg A. J., Koning F., van Furth R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J Immunol. 1991 Dec 1;147(11):3761–3767. [PubMed] [Google Scholar]
- Beekhuizen H., Blokland I., van Furth R. Cross-linking of CD14 molecules on monocytes results in a CD11/CD18- and ICAM-1-dependent adherence to cytokine-stimulated human endothelial cells. J Immunol. 1993 Feb 1;150(3):950–959. [PubMed] [Google Scholar]
- Beekhuizen H., van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993 Oct;54(4):363–378. [PubMed] [Google Scholar]
- Bylock A. L., Gerrity R. G. Visualization of monocyte recruitment into atherosclerotic arteries using fluorescent labelling. Atherosclerosis. 1988 May;71(1):17–25. doi: 10.1016/0021-9150(88)90298-5. [DOI] [PubMed] [Google Scholar]
- Calderon T. M., Factor S. M., Hatcher V. B., Berliner J. A., Berman J. W. An endothelial cell adhesion protein for monocytes recognized by monoclonal antibody IG9. Expression in vivo in inflamed human vessels and atherosclerotic human and Watanabe rabbit vessels. Lab Invest. 1994 Jun;70(6):836–849. [PubMed] [Google Scholar]
- Cavender D. E., Edelbaum D., Welkovich L. Effects of inflammatory cytokines and phorbol esters on the adhesion of U937 cells, a human monocyte-like cell line, to endothelial cell monolayers and extracellular matrix proteins. J Leukoc Biol. 1991 Jun;49(6):566–578. doi: 10.1002/jlb.49.6.566. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., de la Motte C. A. Role of cell surface carbohydrate moieties in monocytic cell adhesion to endothelium in vitro. J Immunol. 1989 Dec 1;143(11):3666–3672. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Tidey R. R., McGregor J. L., Taylor P. R., Poston R. N. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994 May;144(5):952–961. [PMC free article] [PubMed] [Google Scholar]
- Kim J. A., Territo M. C., Wayner E., Carlos T. M., Parhami F., Smith C. W., Haberland M. E., Fogelman A. M., Berliner J. A. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler Thromb. 1994 Mar;14(3):427–433. doi: 10.1161/01.atv.14.3.427. [DOI] [PubMed] [Google Scholar]
- Lauener R. P., Geha R. S., Vercelli D. Engagement of the monocyte surface antigen CD14 induces lymphocyte function-associated antigen-1/intercellular adhesion molecule-1-dependent homotypic adhesion. J Immunol. 1990 Sep 1;145(5):1390–1394. [PubMed] [Google Scholar]
- Lehr H. A., Frei B., Arfors K. E. Vitamin C prevents cigarette smoke-induced leukocyte aggregation and adhesion to endothelium in vivo. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7688–7692. doi: 10.1073/pnas.91.16.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J., Smith J. A., Wilkins G. M., Leake D. S. Oxidation of low density lipoprotein by bovine and porcine aortic endothelial cells and porcine endocardial cells in culture. Atherosclerosis. 1993 Sep;102(2):209–216. doi: 10.1016/0021-9150(93)90163-o. [DOI] [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston R. N., Haskard D. O., Coucher J. R., Gall N. P., Johnson-Tidey R. R. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol. 1992 Mar;140(3):665–673. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sousa A. R., Lane S. J., Nakhosteen J. A., Yoshimura T., Lee T. H., Poston R. N. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994 Feb;10(2):142–147. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G., Hancock W. W. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993 Jun;142(6):1721–1728. [PMC free article] [PubMed] [Google Scholar]
- Wood K. M., Cadogan M. D., Ramshaw A. L., Parums D. V. The distribution of adhesion molecules in human atherosclerosis. Histopathology. 1993 May;22(5):437–444. doi: 10.1111/j.1365-2559.1993.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ulevitch R. J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993 Mar;14(3):121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Tigges A. J., Becker A. E. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992 Dec;141(6):1427–1433. [PMC free article] [PubMed] [Google Scholar]