Abstract
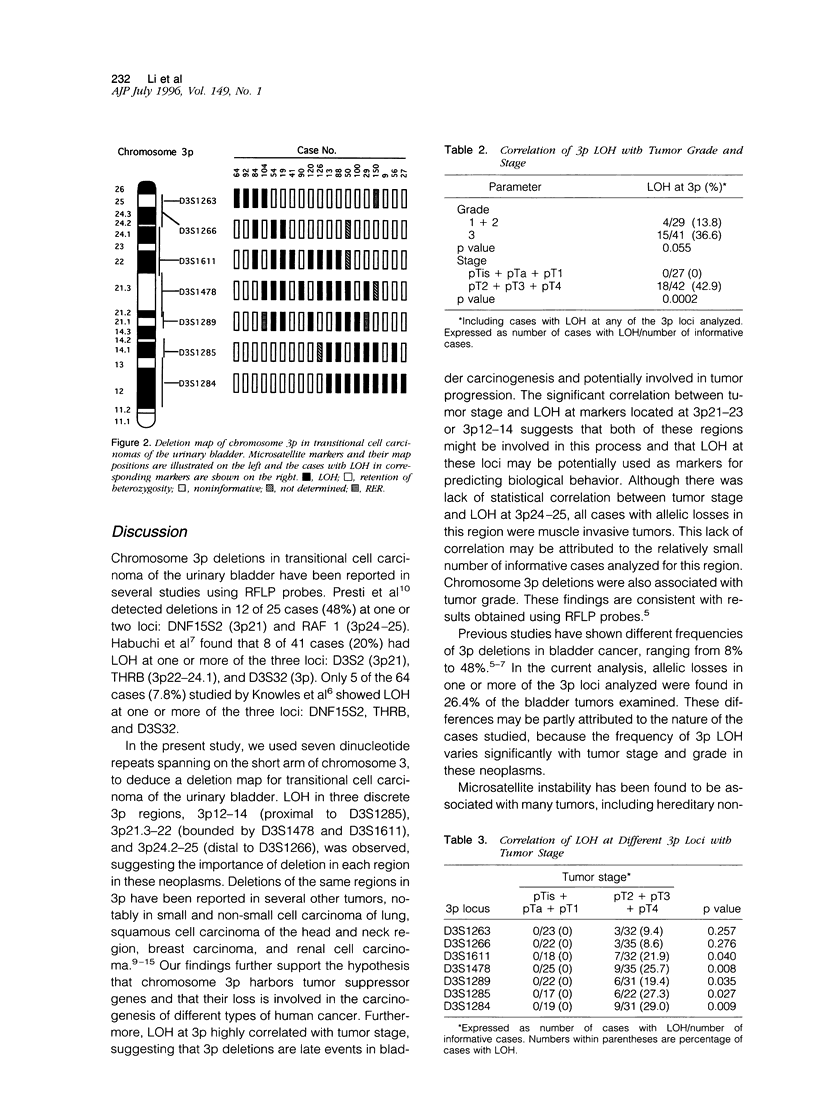
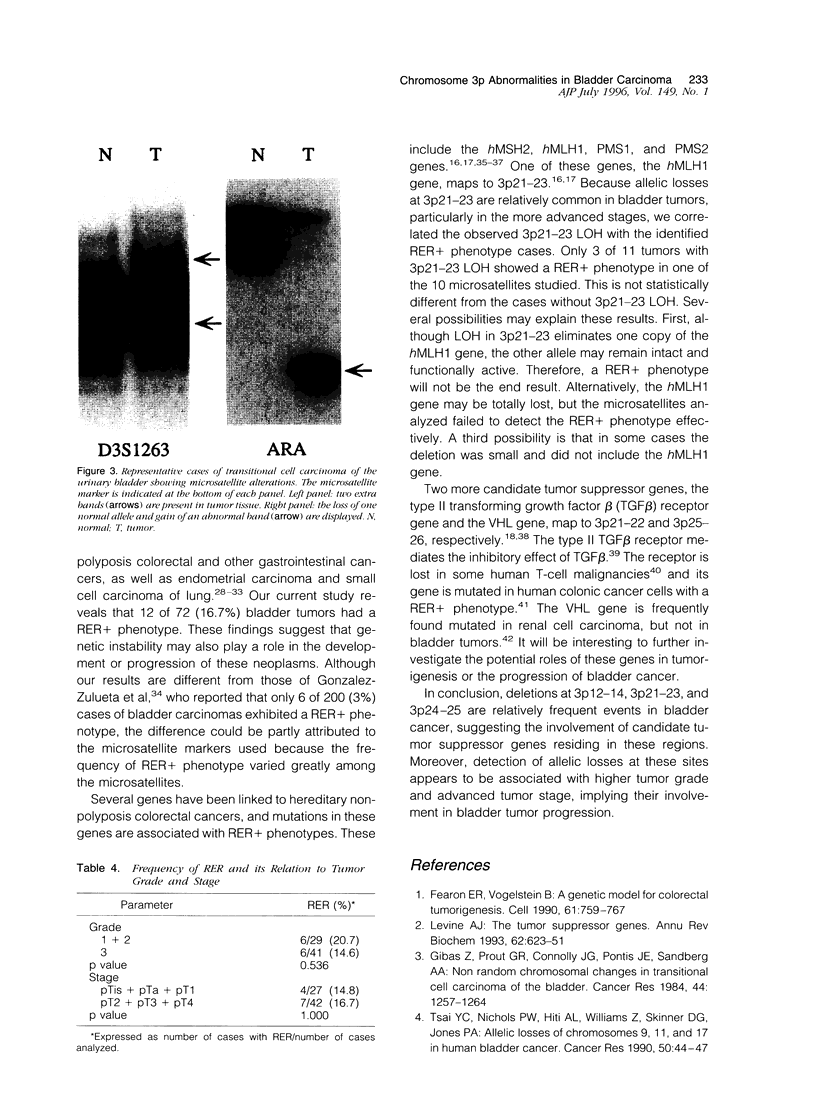
A deletion analysis of chromosome 3 was conducted in 72 cases of transitional cell carcinoma of the urinary bladder using seven microsatellites spanning the 3p arm and two additional microsatellites in 3q. Results showed that 19 of 72 (26.4%) cases had deletions in one or more 3p regions. Two regions of frequent deletion were identified: 3p12-14 and 3p21-23. Less frequent deletions at 3p24.2-25 were also observed. Deletions at 3p were weakly correlated with tumor grade, but strongly with pathological stage. Among 70 cases with histological grade available, 4 of 29 (13.8%) grade 1 and 2 tumors, and 15 of 41 (36.6%) grade 3 tumors showed allelic losses in one or more of the 3p regions studied (P = 0.055). Among 69 cases with pathological stage available, none of 27 superficial carcinomas (pTa, pTis, and pT1) showed 3p deletions, whereas 18 of 42 (42.9%) muscle invasive lesions (pT2, pT3, and pT4) displayed allelic losses at 3p (P < 0.001). In addition, 12 cases showed microsatellite instability, but there was no correlation between abnormalities and tumor grade or stage. No correlation was found between deletions at 3p21-23 and microsatellite instability. In conclusion, deletions at three discrete regions of 3p were identified in bladder carcinoma, suggesting the involvement of candidate tumor suppressor genes residing in these regions. Moreover, detection of allelic losses in these regions was associated with higher tumor grade and more advanced stage, suggesting their potential involvement in bladder tumor progression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Cairns P., Shaw M. E., Knowles M. A. Initiation of bladder cancer may involve deletion of a tumour-suppressor gene on chromosome 9. Oncogene. 1993 Apr;8(4):1083–1085. [PubMed] [Google Scholar]
- Chen L. C., Matsumura K., Deng G., Kurisu W., Ljung B. M., Lerman M. I., Waldman F. M., Smith H. S. Deletion of two separate regions on chromosome 3p in breast cancers. Cancer Res. 1994 Jun 1;54(11):3021–3024. [PubMed] [Google Scholar]
- Chong J. M., Fukayama M., Hayashi Y., Takizawa T., Koike M., Konishi M., Kikuchi-Yanoshita R., Miyaki M. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 1994 Sep 1;54(17):4595–4597. [PubMed] [Google Scholar]
- Dalbagni G., Presti J., Reuter V., Fair W. R., Cordon-Cardo C. Genetic alterations in bladder cancer. Lancet. 1993 Aug 21;342(8869):469–471. doi: 10.1016/0140-6736(93)91595-d. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Gibas Z., Prout G. R., Jr, Connolly J. G., Pontes J. E., Sandberg A. A. Nonrandom chromosomal changes in transitional cell carcinoma of the bladder. Cancer Res. 1984 Mar;44(3):1257–1264. [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Ruppert J. M., Tokino K., Tsai Y. C., Spruck C. H., 3rd, Miyao N., Nichols P. W., Hermann G. G., Horn T., Steven K. Microsatellite instability in bladder cancer. Cancer Res. 1993 Dec 1;53(23):5620–5623. [PubMed] [Google Scholar]
- Habuchi T., Ogawa O., Kakehi Y., Ogura K., Koshiba M., Hamazaki S., Takahashi R., Sugiyama T., Yoshida O. Accumulated allelic losses in the development of invasive urothelial cancer. Int J Cancer. 1993 Feb 20;53(4):579–584. doi: 10.1002/ijc.2910530409. [DOI] [PubMed] [Google Scholar]
- Johnson B. E., Sakaguchi A. Y., Gazdar A. F., Minna J. D., Burch D., Marshall A., Naylor S. L. Restriction fragment length polymorphism studies show consistent loss of chromosome 3p alleles in small cell lung cancer patients' tumors. J Clin Invest. 1988 Aug;82(2):502–507. doi: 10.1172/JCI113624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Cavaille-Coll M. W., Gertz R., Massagué J., Cheifetz S., George D. Loss of receptors for transforming growth factor beta in human T-cell malignancies. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6002–6006. doi: 10.1073/pnas.91.13.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killary A. M., Wolf M. E., Giambernardi T. A., Naylor S. L. Definition of a tumor suppressor locus within human chromosome 3p21-p22. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10877–10881. doi: 10.1073/pnas.89.22.10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. A., Elder P. A., Williamson M., Cairns J. P., Shaw M. E., Law M. G. Allelotype of human bladder cancer. Cancer Res. 1994 Jan 15;54(2):531–538. [PubMed] [Google Scholar]
- Koi M., Umar A., Chauhan D. P., Cherian S. P., Carethers J. M., Kunkel T. A., Boland C. R. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994 Aug 15;54(16):4308–4312. [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- Maestro R., Gasparotto D., Vukosavljevic T., Barzan L., Sulfaro S., Boiocchi M. Three discrete regions of deletion at 3p in head and neck cancers. Cancer Res. 1993 Dec 1;53(23):5775–5779. [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Mathew S., Murty V. V., Cheifetz S., George D., Massagué J., Chaganti R. S. Transforming growth factor receptor gene TGFBR2 maps to human chromosome band 3p22. Genomics. 1994 Mar 1;20(1):114–115. doi: 10.1006/geno.1994.1134. [DOI] [PubMed] [Google Scholar]
- Meltzer S. J., Yin J., Manin B., Rhyu M. G., Cottrell J., Hudson E., Redd J. L., Krasna M. J., Abraham J. M., Reid B. J. Microsatellite instability occurs frequently and in both diploid and aneuploid cell populations of Barrett's-associated esophageal adenocarcinomas. Cancer Res. 1994 Jul 1;54(13):3379–3382. [PubMed] [Google Scholar]
- Merlo A., Mabry M., Gabrielson E., Vollmer R., Baylin S. B., Sidransky D. Frequent microsatellite instability in primary small cell lung cancer. Cancer Res. 1994 Apr 15;54(8):2098–2101. [PubMed] [Google Scholar]
- Morita R., Ishikawa J., Tsutsumi M., Hikiji K., Tsukada Y., Kamidono S., Maeda S., Nakamura Y. Allelotype of renal cell carcinoma. Cancer Res. 1991 Feb 1;51(3):820–823. [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Orlow I., Lianes P., Lacombe L., Dalbagni G., Reuter V. E., Cordon-Cardo C. Chromosome 9 allelic losses and microsatellite alterations in human bladder tumors. Cancer Res. 1994 Jun 1;54(11):2848–2851. [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Cordon-Cardo C., Mazumdar M., Fair W. R., Jhanwar S. C. Allelic deletions in renal tumors: histopathological correlations. Cancer Res. 1993 Dec 1;53(23):5780–5783. [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Galan T., Fair W. R., Cordon-Cardo C. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991 Oct 1;51(19):5405–5409. [PubMed] [Google Scholar]
- Risinger J. I., Berchuck A., Kohler M. F., Watson P., Lynch H. T., Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993 Nov 1;53(21):5100–5103. [PubMed] [Google Scholar]
- Sidransky D., Messing E. Molecular genetics and biochemical mechanisms in bladder cancer. Oncogenes, tumor suppressor genes, and growth factors. Urol Clin North Am. 1992 Nov;19(4):629–639. [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Tsai Y. C., Nichols P. W., Hiti A. L., Williams Z., Skinner D. G., Jones P. A. Allelic losses of chromosomes 9, 11, and 17 in human bladder cancer. Cancer Res. 1990 Jan 1;50(1):44–47. [PubMed] [Google Scholar]
- Wu C. L., Sloan P., Read A. P., Harris R., Thakker N. Deletion mapping on the short arm of chromosome 3 in squamous cell carcinoma of the oral cavity. Cancer Res. 1994 Dec 15;54(24):6484–6488. [PubMed] [Google Scholar]
- Yokoyama S., Yamakawa K., Tsuchiya E., Murata M., Sakiyama S., Nakamura Y. Deletion mapping on the short arm of chromosome 3 in squamous cell carcinoma and adenocarcinoma of the lung. Cancer Res. 1992 Feb 15;52(4):873–877. [PubMed] [Google Scholar]




