Abstract
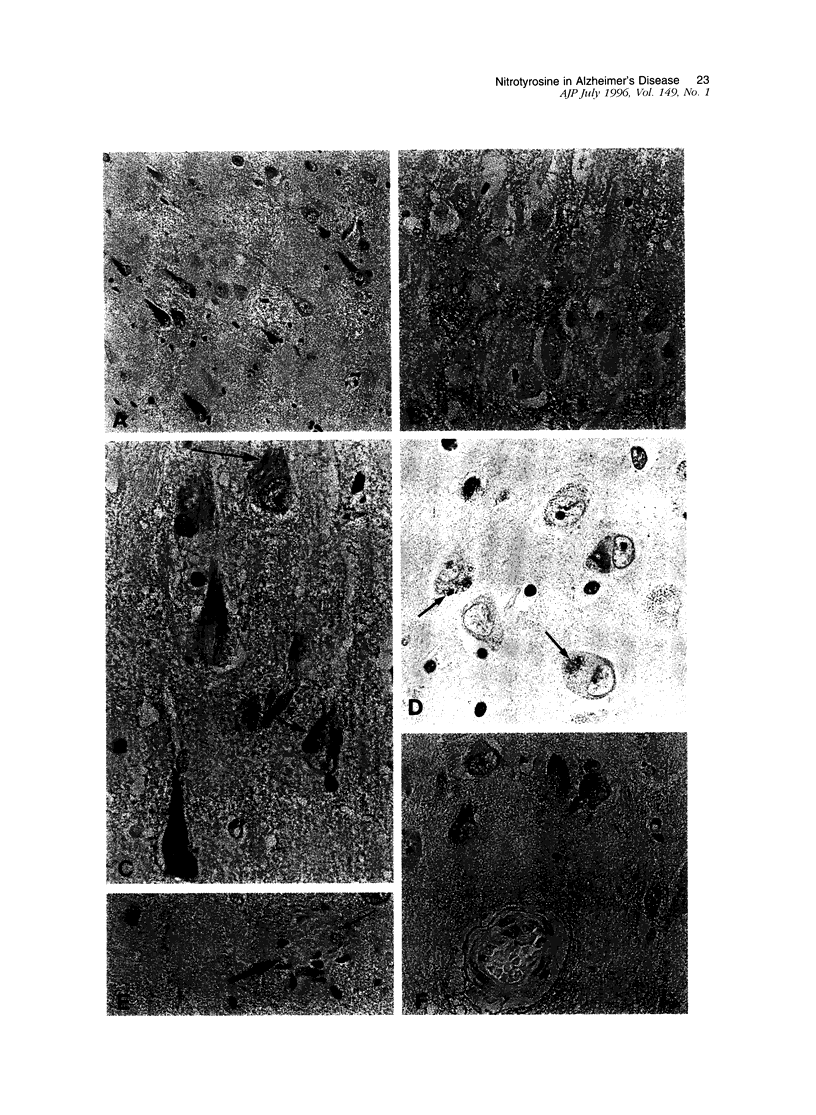
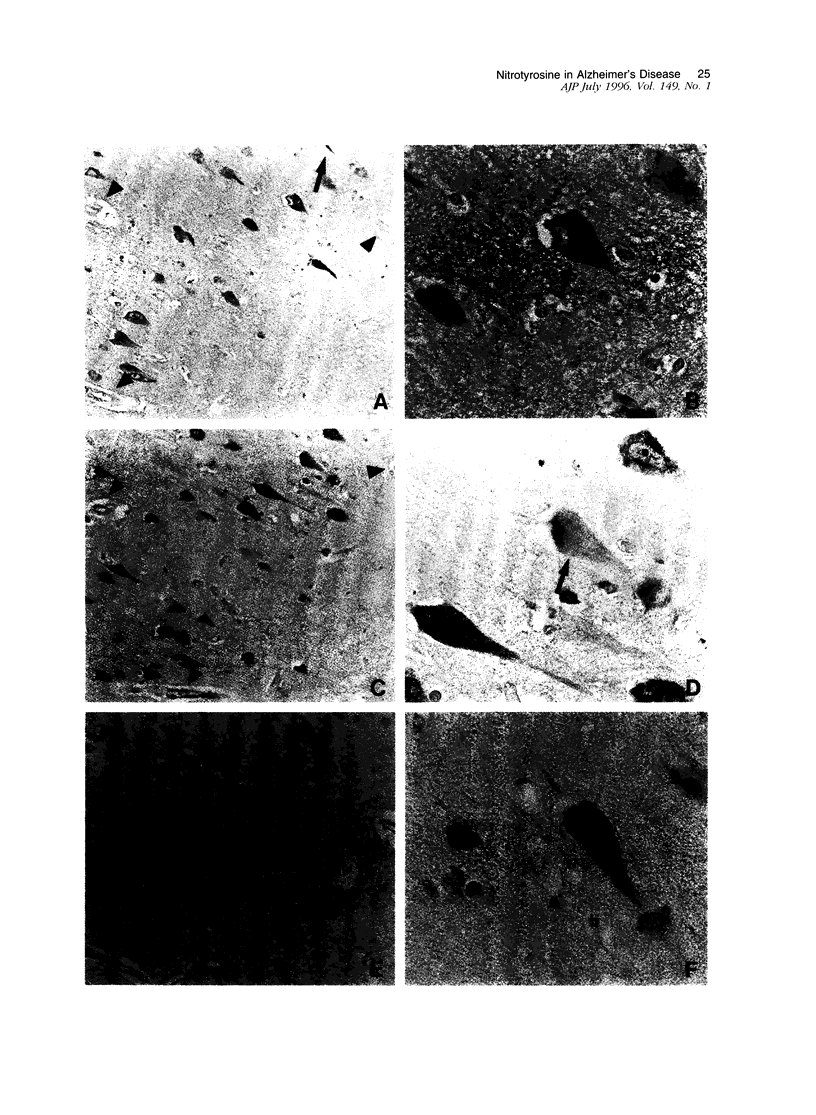
Oxidative stress has been proposed as a pathogenetic mechanism in Alzheimer's disease. One mechanism of oxidative damage is the nitration of tyrosine residues in proteins, mediated by peroxynitrite breakdown. Peroxynitrite, a reaction product of nitric oxide and superoxide radicals, has been implicated in N-methyl-D-aspartate receptor-mediated excitotoxic damage. Reported evidence of oxidative stress in Alzheimer's disease includes increased iron, alterations in protective enzymes, and markers of oxidative damage to proteins and lipids. In this report, we demonstrate the presence of nitrotyrosine in neurofibrillary tangles of Alzheimer's disease. Nitrotyrosine was not detected in controls lacking neurofibrillary tangles. Immunolabeling was demonstrated to be specific nitrotyrosine in a series of control experiments. These observations link oxidative stress with a key pathological lesion of Alzheimer's disease, the neurofibrillary tangle, and demonstrate a pathogenetic mechanism in common with the other major neurodegenerative diseases of aging, Parkinson's disease and amyotrophic lateral sclerosis. These findings further implicate nitric oxide expression and excitotoxicity in the pathogenesis of cell death in Alzheimer's disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Ischiropoulos H., Zhu L., van der Woerd M., Smith C., Chen J., Harrison J., Martin J. C., Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Bouras C., Hof P. R., Giannakopoulos P., Michel J. P., Morrison J. H. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994 Mar-Apr;4(2):138–150. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter D. T., Carter C. J., Wells F. R., Javoy-Agid F., Agid Y., Lees A., Jenner P., Marsden C. D. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989 Feb;52(2):381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Dyrks E., Hartmann T., Masters C., Beyreuther K. Amyloidogenicity of beta A4 and beta A4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J Biol Chem. 1992 Sep 5;267(25):18210–18217. [PubMed] [Google Scholar]
- Fahn S., Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992 Dec;32(6):804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Boulton C. L. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P., Hof P. R., Mottier S., Michel J. P., Bouras C. Neuropathological changes in the cerebral cortex of 1258 cases from a geriatric hospital: retrospective clinicopathological evaluation of a 10-year autopsy population. Acta Neuropathol. 1994;87(5):456–468. doi: 10.1007/BF00294172. [DOI] [PubMed] [Google Scholar]
- Good P. F., Olanow C. W., Perl D. P. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson's disease: a LAMMA study. Brain Res. 1992 Oct 16;593(2):343–346. doi: 10.1016/0006-8993(92)91334-b. [DOI] [PubMed] [Google Scholar]
- Good P. F., Perl D. P., Bierer L. M., Schmeidler J. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol. 1992 Mar;31(3):286–292. doi: 10.1002/ana.410310310. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Hof P. R., Cox K., Morrison J. H. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990 Nov 1;301(1):44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kooy N. W., Royall J. A., Ye Y. Z., Kelly D. R., Beckman J. S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995 Apr;151(4):1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M., Culcasi M., Gaven F., Pietri S., Bockaert J. Nitric oxide, superoxide and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacology. 1993 Nov;32(11):1259–1266. doi: 10.1016/0028-3908(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Malinski T., Bailey F., Zhang Z. G., Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1993 May;13(3):355–358. doi: 10.1038/jcbfm.1993.48. [DOI] [PubMed] [Google Scholar]
- Mann V. M., Cooper J. M., Daniel S. E., Srai K., Jenner P., Marsden C. D., Schapira A. H. Complex I, iron, and ferritin in Parkinson's disease substantia nigra. Ann Neurol. 1994 Dec;36(6):876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P., MacGarvey U., Kaufman A. E., Koontz D., Shoffner J. M., Wallace D. C., Beal M. F. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993 Oct;34(4):609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- Mirra S. S., Heyman A., McKeel D., Sumi S. M., Crain B. J., Brownlee L. M., Vogel F. S., Hughes J. P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Pappolla M. A., Omar R. A., Kim K. S., Robakis N. K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992 Mar;140(3):621–628. [PMC free article] [PubMed] [Google Scholar]
- Reif D. W., Simmons R. D. Nitric oxide mediates iron release from ferritin. Arch Biochem Biophys. 1990 Dec;283(2):537–541. doi: 10.1016/0003-9861(90)90680-w. [DOI] [PubMed] [Google Scholar]
- Riederer P., Sofic E., Rausch W. D., Schmidt B., Reynolds G. P., Jellinger K., Youdim M. B. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989 Feb;52(2):515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Robberecht W., Sapp P., Viaene M. K., Rosen D., McKenna-Yasek D., Haines J., Horvitz R., Theys P., Brown R., Jr Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1994 Jan;62(1):384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Schreck R., Albermann K., Baeuerle P. A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17(4):221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Rudnicka-Nawrot M., Richey P. L., Praprotnik D., Mulvihill P., Miller C. A., Sayre L. M., Perry G. Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer's disease. J Neurochem. 1995 Jun;64(6):2660–2666. doi: 10.1046/j.1471-4159.1995.64062660.x. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Sayre L. M., Monnier V. M., Perry G. Radical AGEing in Alzheimer's disease. Trends Neurosci. 1995 Apr;18(4):172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- Somerville M. J., Percy M. E., Bergeron C., Yoong L. K., Grima E. A., McLachlan D. R. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991 Jan;9(1-2):1–8. doi: 10.1016/0169-328x(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Subbarao K. V., Richardson J. S., Ang L. C. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990 Jul;55(1):342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- Troncoso J. C., Costello A., Watson A. L., Jr, Johnson G. V. In vitro polymerization of oxidized tau into filaments. Brain Res. 1993 Jun 11;613(2):313–316. doi: 10.1016/0006-8993(93)90918-d. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Munthasser S., Fraser P. E., Percy M. E., Rainero I., Vaula G., Pinessi L., Bergamini L., Vignocchi G., McLachlan D. R. Analysis of the functional effects of a mutation in SOD1 associated with familial amyotrophic lateral sclerosis. Neuron. 1994 Sep;13(3):727–736. doi: 10.1016/0896-6273(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Yan S. F., Chen X., Fu J., Chen M., Kuppusamy P., Smith M. A., Perry G., Godman G. C., Nawroth P. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995 Jul;1(7):693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]