Abstract
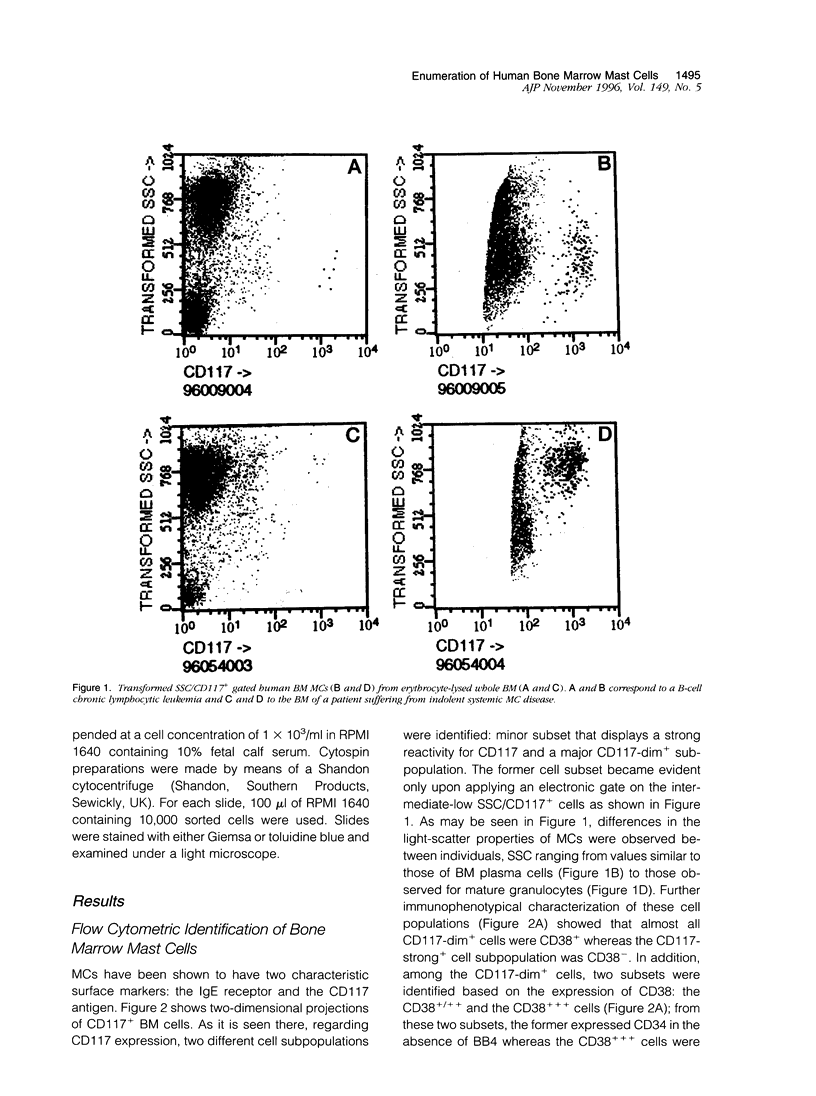
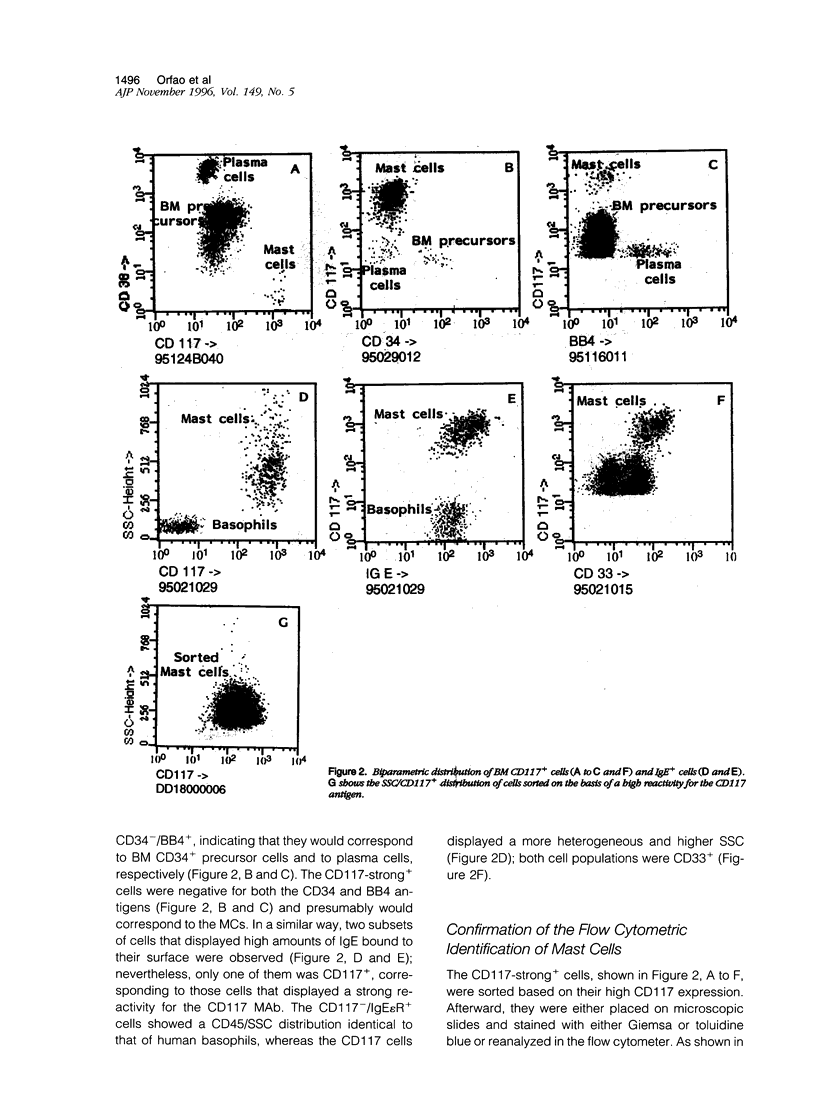
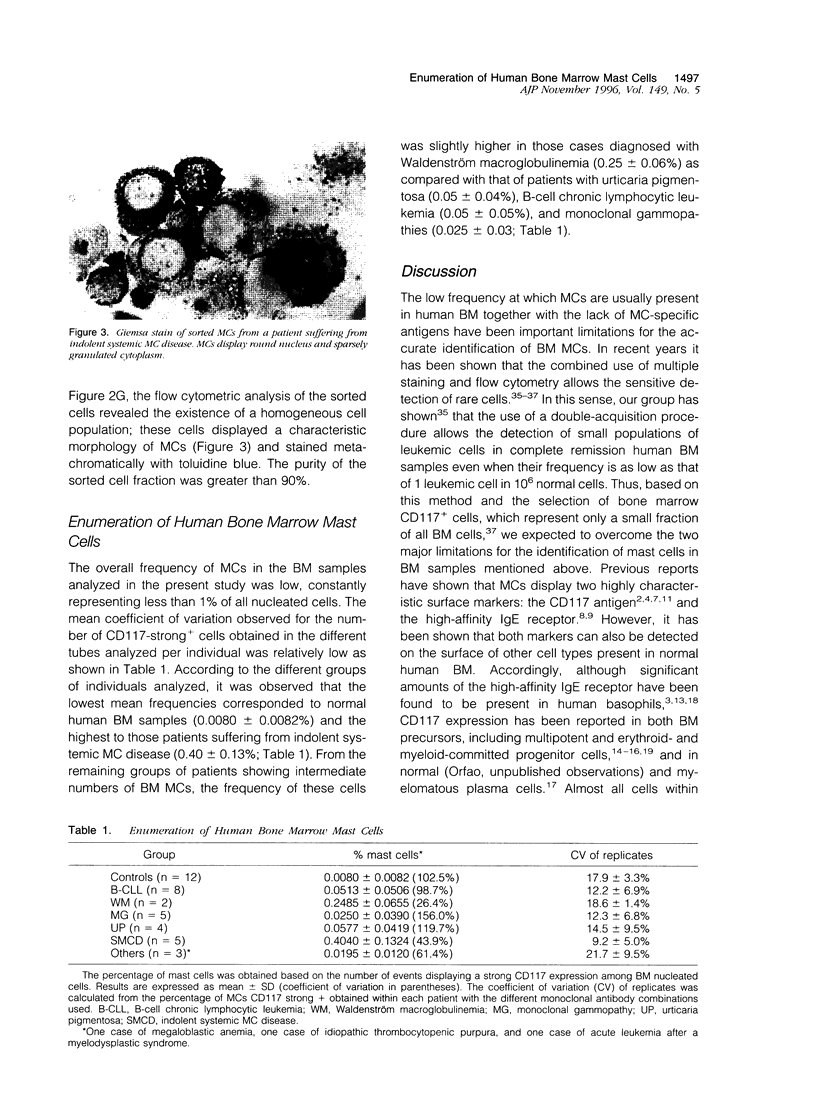
In the present paper we have used a three-color immunofluorescence procedure combined with flow cytometry cell analysis and sorting for the identification and enumeration of human mast cells in both normal and pathological bone marrow samples. Our results show that bone marrow mast cells are clearly identifiable on the basis of their light-scatter properties and strong CD117 expression. These cells were negative for the CD34, CD38, and BB4 antigens. In addition, they were CD33+ and displayed a high reactivity for the anti-IgE monoclonal antibody. The identity of the CD117-strong+ cells (mast cells) was confirmed by both microscopic examination and flow cytometry analysis. The overall frequency of mast cells in the bone marrow samples analyzed in the present study was constantly lower than 1%. The lowest frequencies corresponded to normal human bone marrow samples (0.0080 +/- 0.0082%) and the highest to those patients suffering from indolent systemic mast cell disease (0.40 +/- 0.13%). In summary, our results show that the identification and enumeration of bone marrow mast cells can be achieved using multiparametric flow cytometry. Moreover, once identified, mast cells are suitable for being characterized from the phenotypic and the functional point of view, facilitating the comparison between normal and abnormal mast cells.
Full text
PDF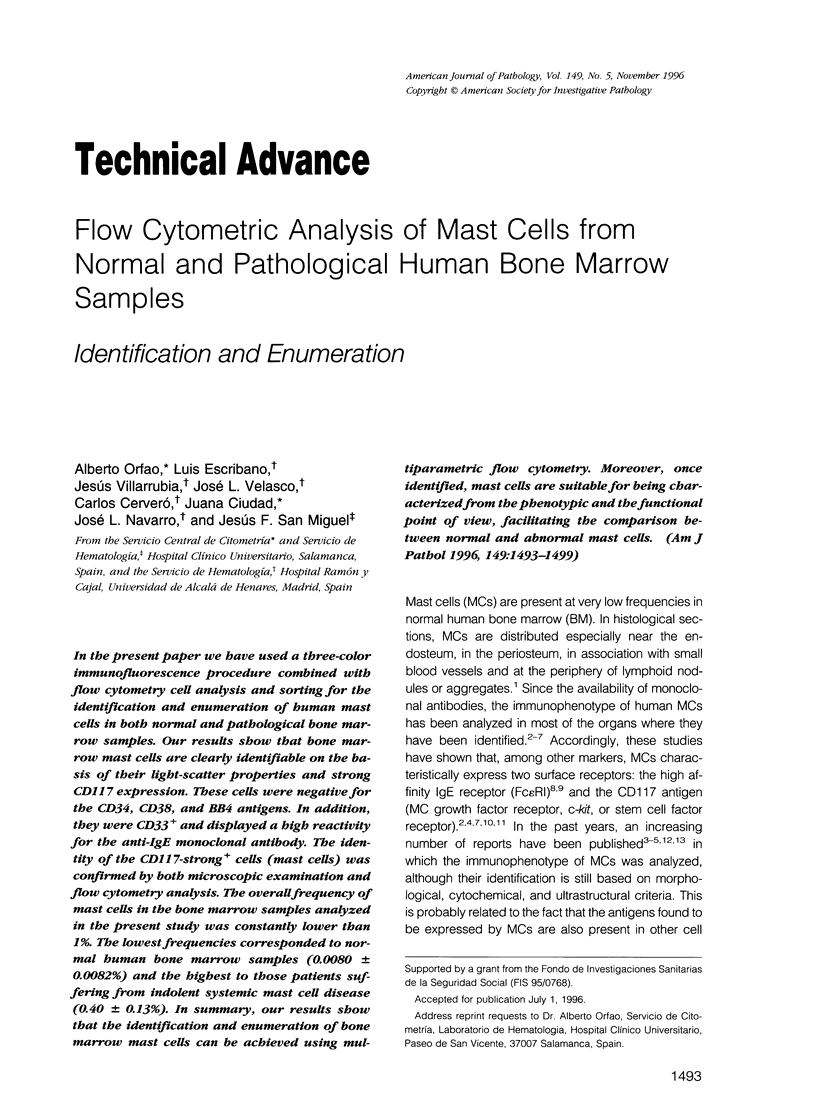



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltomaa S., Lipponen P., Papinaho S., Kosma V. M. Mast cells in breast cancer. Anticancer Res. 1993 May-Jun;13(3):785–788. [PubMed] [Google Scholar]
- Ashman L. K., Cambareri A. C., To L. B., Levinsky R. J., Juttner C. A. Expression of the YB5.B8 antigen (c-kit proto-oncogene product) in normal human bone marrow. Blood. 1991 Jul 1;78(1):30–37. [PubMed] [Google Scholar]
- Brando B., Sommaruga E. Nationwide quality control trial on lymphocyte immunophenotyping and flow cytometer performance in Italy. Cytometry. 1993;14(3):294–306. doi: 10.1002/cyto.990140310. [DOI] [PubMed] [Google Scholar]
- Bühring H. J., Ullrich A., Schaudt K., Müller C. A., Busch F. W. The product of the proto-oncogene c-kit (P145c-kit) is a human bone marrow surface antigen of hemopoietic precursor cells which is expressed on a subset of acute non-lymphoblastic leukemic cells. Leukemia. 1991 Oct;5(10):854–860. [PubMed] [Google Scholar]
- Carr N. J., Warren A. Y. Mast cell numbers in melanocytic naevi and cutaneous neurofibromas. J Clin Pathol. 1993 Jan;46(1):86–87. doi: 10.1136/jcp.46.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J., Smith P. J. A quantitative study of mast cells in Hodgkin's disease. J Clin Pathol. 1984 May;37(5):519–522. doi: 10.1136/jcp.37.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano L., Orfao A., Villarrubia J., Cerveró C., Velasco J. L., Martín F., San Miguel J. F., Navarro J. L. Expression of lymphoid-associated antigens in mast cells: report of a case of systemic mast cell disease. Br J Haematol. 1995 Dec;91(4):941–943. doi: 10.1111/j.1365-2141.1995.tb05417.x. [DOI] [PubMed] [Google Scholar]
- Froese A. Receptors for IgE on mast cells and basophils. Prog Allergy. 1984;34:142–187. [PubMed] [Google Scholar]
- Födinger M., Fritsch G., Winkler K., Emminger W., Mitterbauer G., Gadner H., Valent P., Mannhalter C. Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood. 1994 Nov 1;84(9):2954–2959. [PubMed] [Google Scholar]
- Gane P., Pecquet C., Lambin P., Abuaf N., Leynadier F., Rouger P. Flow cytometric evaluation of human basophils. Cytometry. 1993;14(3):344–348. doi: 10.1002/cyto.990140316. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Verwer B., Houck D., Recktenwald D. Detection of rare cells at a frequency of one per million by flow cytometry. Cytometry. 1993;14(5):519–526. doi: 10.1002/cyto.990140511. [DOI] [PubMed] [Google Scholar]
- Guo C. B., Kagey-Sobotka A., Lichtenstein L. M., Bochner B. S. Immunophenotyping and functional analysis of purified human uterine mast cells. Blood. 1992 Feb 1;79(3):708–712. [PubMed] [Google Scholar]
- Irani A. M., Gruber B. L., Kaufman L. D., Kahaleh M. B., Schwartz L. B. Mast cell changes in scleroderma. Presence of MCT cells in the skin and evidence of mast cell activation. Arthritis Rheum. 1992 Aug;35(8):933–939. doi: 10.1002/art.1780350813. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- JOHNSTONE J. M. The appearance and significance of tissue mast cells in human bone marrow. J Clin Pathol. 1954 Nov;7(4):275–280. doi: 10.1136/jcp.7.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper C. S., Tharp M. D. Quantification of cutaneous mast cells using morphometric point counting and a conjugated avidin stain. J Am Acad Dermatol. 1987 Feb;16(2 Pt 1):326–331. doi: 10.1016/s0190-9622(87)70044-9. [DOI] [PubMed] [Google Scholar]
- Kirby J. G., Hargreave F. E., Gleich G. J., O'Byrne P. M. Bronchoalveolar cell profiles of asthmatic and nonasthmatic subjects. Am Rev Respir Dis. 1987 Aug;136(2):379–383. doi: 10.1164/ajrccm/136.2.379. [DOI] [PubMed] [Google Scholar]
- Lanza F., Moretti S., Papa S., Malavasi F., Castoldi G. Report on the Fifth International Workshop on Human Leukocyte Differentiation Antigens, Boston, November 3-7, 1993. Haematologica. 1994 Jul-Aug;79(4):374–386. [PubMed] [Google Scholar]
- Lemoli R. M., Fortuna A., Grande A., Gamberi B., Bonsi L., Fogli M., Amabile M., Cavo M., Ferrari S., Tura S. Expression and functional role of c-kit ligand (SCF) in human multiple myeloma cells. Br J Haematol. 1994 Dec;88(4):760–769. doi: 10.1111/j.1365-2141.1994.tb05115.x. [DOI] [PubMed] [Google Scholar]
- Levin L. A., Albert D. M., Johnson D. Mast cells in human optic nerve. Invest Ophthalmol Vis Sci. 1993 Oct;34(11):3147–3153. [PubMed] [Google Scholar]
- Macedo A., Orfao A., Martínez A., Vidriales M. B., Valverde B., López-Berges M. C., San Miguel J. F. Immunophenotype of c-kit cells in normal human bone marrow: implications for the detection of minimal residual disease in AML. Br J Haematol. 1995 Feb;89(2):338–341. doi: 10.1111/j.1365-2141.1995.tb03309.x. [DOI] [PubMed] [Google Scholar]
- McKenna M. J. Histomorphometric study of mast cells in normal bone, osteoporosis and mastocytosis using a new stain. Calcif Tissue Int. 1994 Oct;55(4):257–259. doi: 10.1007/BF00310402. [DOI] [PubMed] [Google Scholar]
- Mirowski G., Austen K. F., Chiang L., Horan R. F., Sheffer A. L., Weidner N., Murphy G. F. Characterization of cellular dermal infiltrates in human cutaneous mastocytosis. Lab Invest. 1990 Jul;63(1):52–62. [PubMed] [Google Scholar]
- Orfao A., Ciudad J., Lopez-Berges M. C., Lopez A., Vidriales B., Caballero M. D., Valverde B., Gonzalez M., San Miguel J. F. Acute lymphoblastic leukemia (ALL): detection of minimal residual disease (MRD) at flow cytometry. Leuk Lymphoma. 1994;13 (Suppl 1):87–90. doi: 10.3109/10428199409052682. [DOI] [PubMed] [Google Scholar]
- Orfäo A., García-Sanz R., López-Berges M. C., Belén Vidriales M., González M., Caballero M. D., San Miguel J. F. A new method for the analysis of plasma cell DNA content in multiple myeloma samples using a CD38/propidium iodide double staining technique. Cytometry. 1994 Dec 1;17(4):332–339. doi: 10.1002/cyto.990170409. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Broudy V. C., Zsebo K. M. Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: functional properties and composite antigenic profile. Blood. 1991 Sep 15;78(6):1403–1412. [PubMed] [Google Scholar]
- Prokocimer M., Polliack A. Increased bone marrow mast cells in preleukemic syndromes, acute leukemia, and lymphoproliferative disorders. Am J Clin Pathol. 1981 Jan;75(1):34–38. doi: 10.1093/ajcp/75.1.34. [DOI] [PubMed] [Google Scholar]
- Ratajczak M. Z., Luger S. M., Gewirtz A. M. The c-kit proto-oncogene in normal and malignant human hematopoiesis. Int J Cell Cloning. 1992 Jul;10(4):205–214. doi: 10.1002/stem.5530100403. [DOI] [PubMed] [Google Scholar]
- Rudolph M. I., Reinicke K., Cruz M. A., Gallardo V., Gonzalez C., Bardisa L. Distribution of mast cells and the effect of their mediators on contractility in human myometrium. Br J Obstet Gynaecol. 1993 Dec;100(12):1125–1130. doi: 10.1111/j.1471-0528.1993.tb15178.x. [DOI] [PubMed] [Google Scholar]
- Sale G. E., Marmont P. Marrow mast cell counts do not predict bone marrow graft rejection. Hum Pathol. 1981 Jul;12(7):605–608. doi: 10.1016/s0046-8177(81)80043-3. [DOI] [PubMed] [Google Scholar]
- Sperr W. R., Bankl H. C., Mundigler G., Klappacher G., Grossschmidt K., Agis H., Simon P., Laufer P., Imhof M., Radaszkiewicz T. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood. 1994 Dec 1;84(11):3876–3884. [PubMed] [Google Scholar]
- Strobel S., Busuttil A., Ferguson A. Human intestinal mucosal mast cells: expanded population in untreated coeliac disease. Gut. 1983 Mar;24(3):222–227. doi: 10.1136/gut.24.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstappen L. W., Hollander Z., Meiners H., Loken M. R. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990 Aug;48(2):138–148. doi: 10.1002/jlb.48.2.138. [DOI] [PubMed] [Google Scholar]
- Terstappen L. W., Johnsen S., Segers-Nolten I. M., Loken M. R. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood. 1990 Nov 1;76(9):1739–1747. [PubMed] [Google Scholar]
- Valent P., Ashman L. K., Hinterberger W., Eckersberger F., Majdic O., Lechner K., Bettelheim P. Mast cell typing: demonstration of a distinct hematopoietic cell type and evidence for immunophenotypic relationship to mononuclear phagocytes. Blood. 1989 May 15;73(7):1778–1785. [PubMed] [Google Scholar]
- Valent P., Bettelheim P. Cell surface structures on human basophils and mast cells: biochemical and functional characterization. Adv Immunol. 1992;52:333–423. doi: 10.1016/s0065-2776(08)60879-2. [DOI] [PubMed] [Google Scholar]
- Valent P., Majdic O., Maurer D., Bodger M., Muhm M., Bettelheim P. Further characterization of surface membrane structures expressed on human basophils and mast cells. Int Arch Allergy Appl Immunol. 1990;91(2):198–203. doi: 10.1159/000235115. [DOI] [PubMed] [Google Scholar]
- Valent P. The phenotype of human eosinophils, basophils, and mast cells. J Allergy Clin Immunol. 1994 Dec;94(6 Pt 2):1177–1183. doi: 10.1016/0091-6749(94)90329-8. [DOI] [PubMed] [Google Scholar]
- Yoo D., Lessin L. S. Bone marrow mast cell content in preleukemic syndrome. Am J Med. 1982 Oct;73(4):539–542. doi: 10.1016/0002-9343(82)90333-3. [DOI] [PubMed] [Google Scholar]
- Yoo D., Lessin L. S., Jensen W. N. Bone-marrow mast cells in lymphoproliferative disorders. Ann Intern Med. 1978 Jun;88(6):753–757. doi: 10.7326/0003-4819-88-6-753. [DOI] [PubMed] [Google Scholar]
- de Gennes C., Kuntz D., de Vernejoul M. C. Bone mastocytosis. A report of nine cases with a bone histomorphometric study. Clin Orthop Relat Res. 1992 Jun;(279):281–291. [PubMed] [Google Scholar]




