Abstract
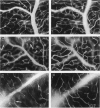
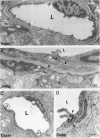
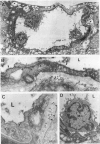
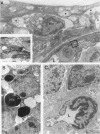
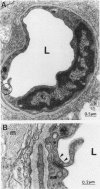
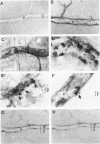
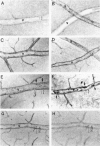
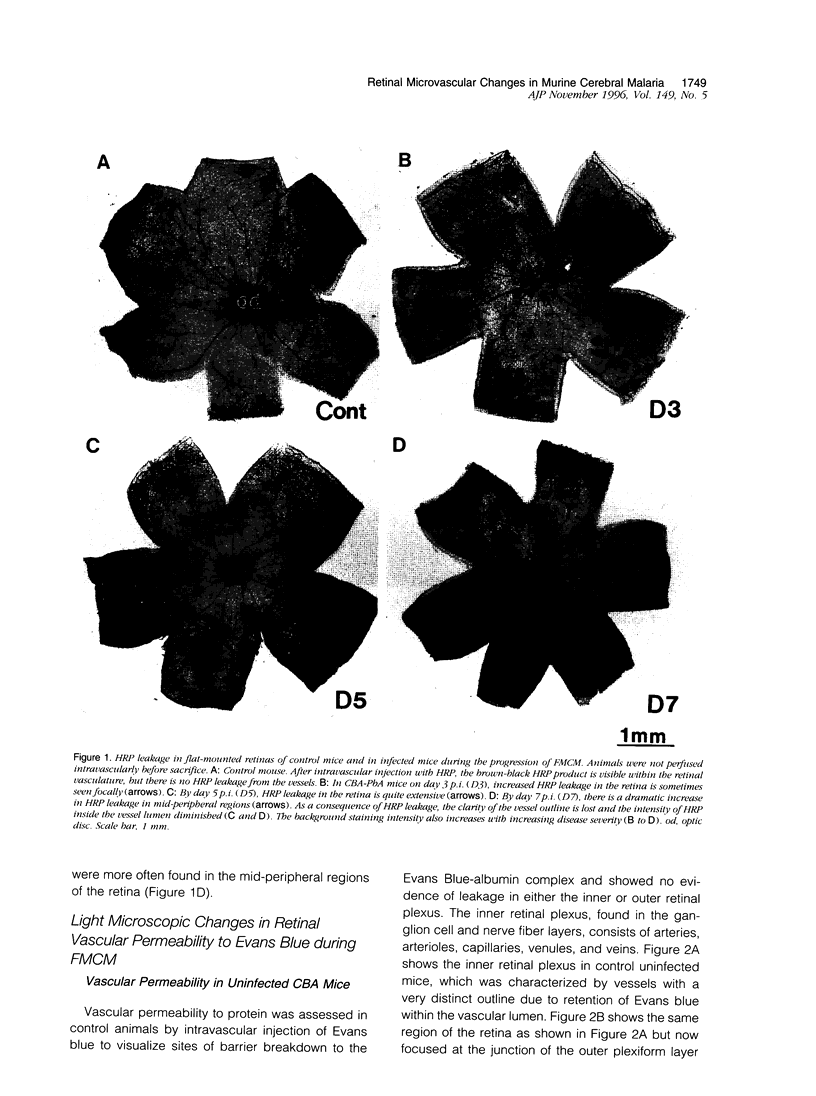
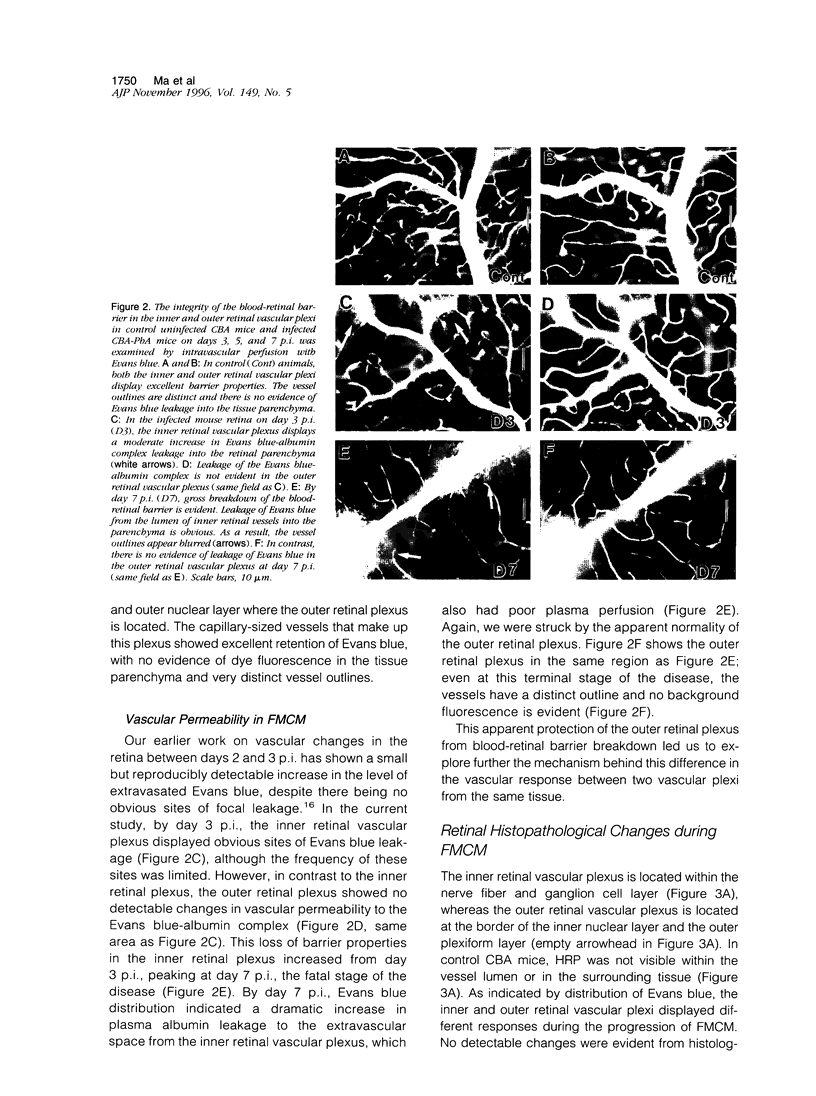
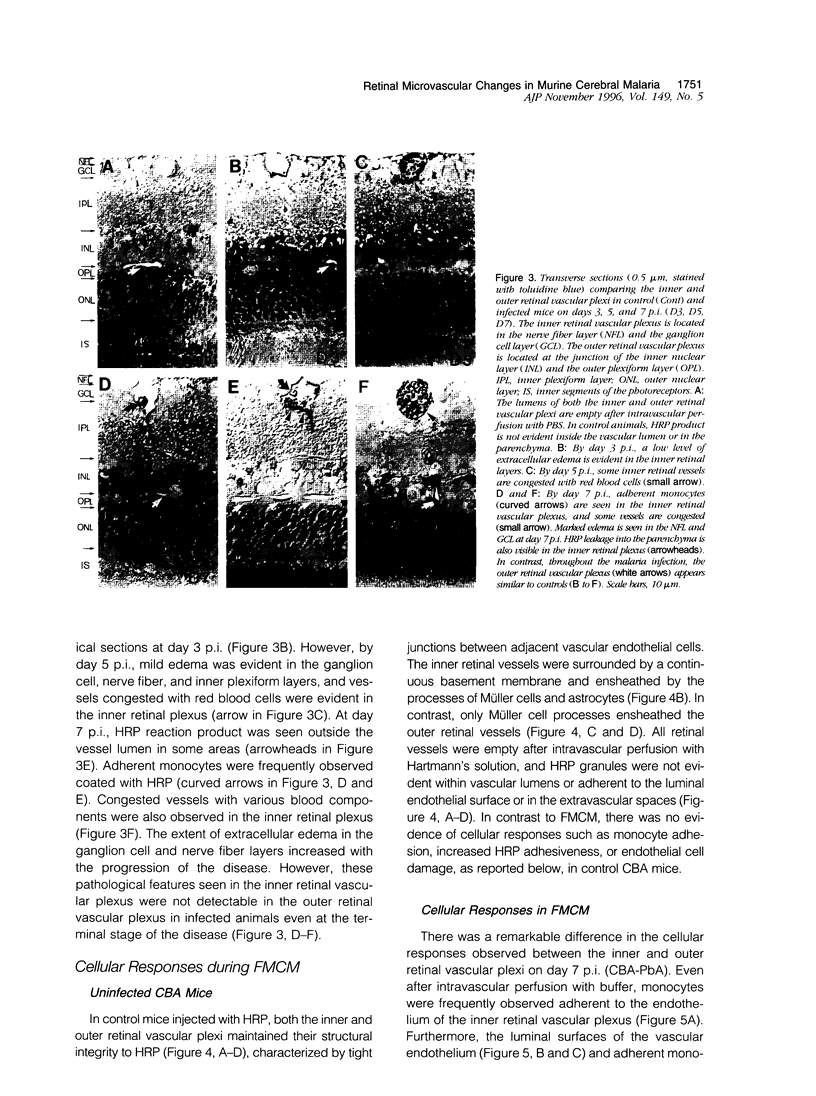
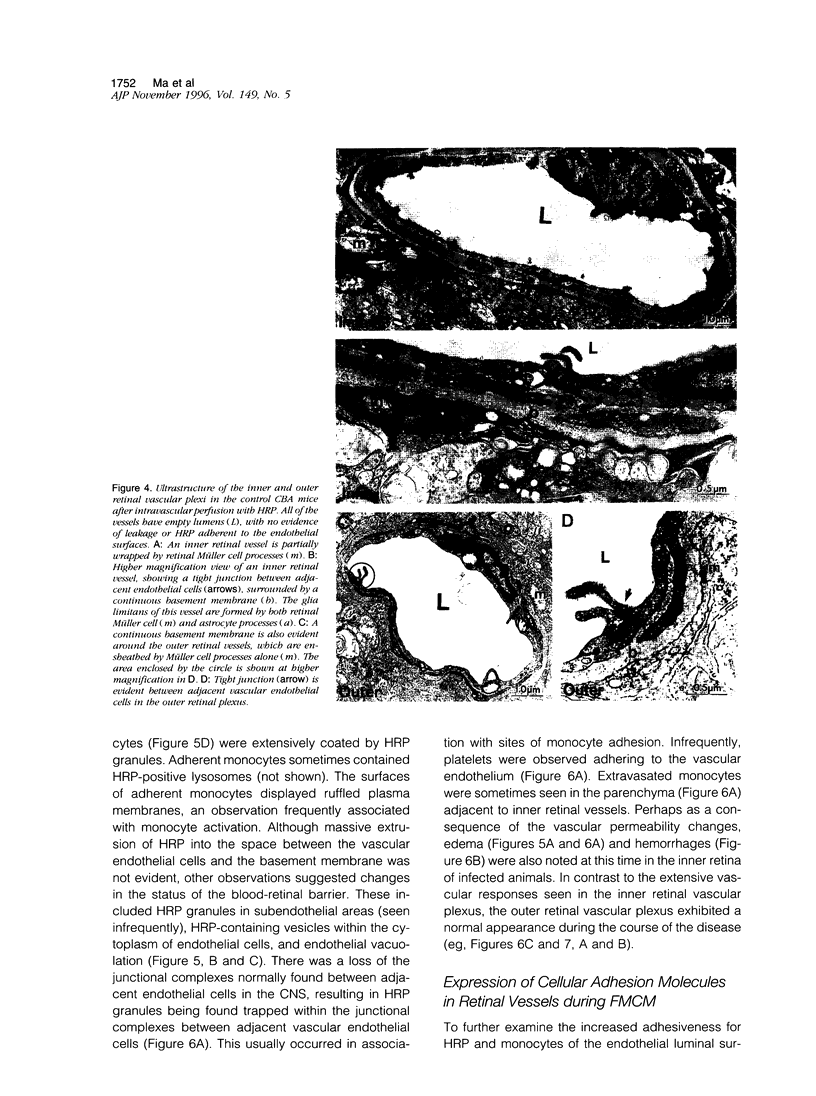
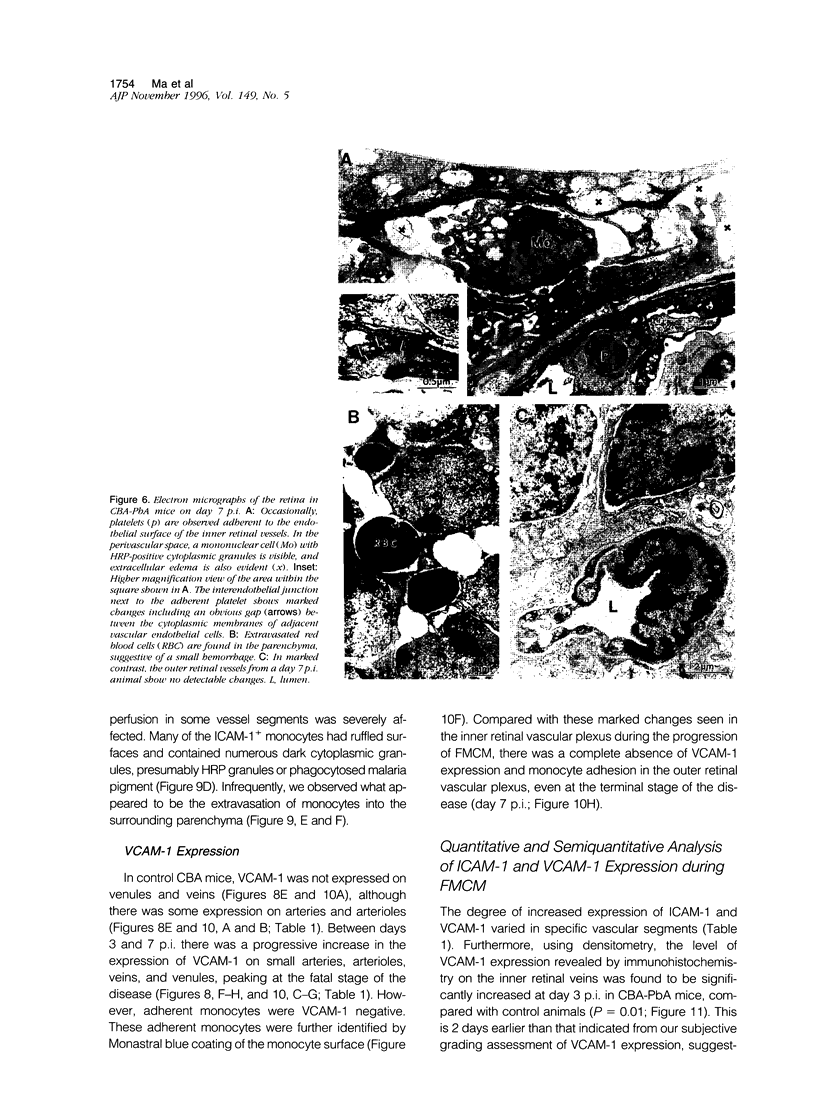
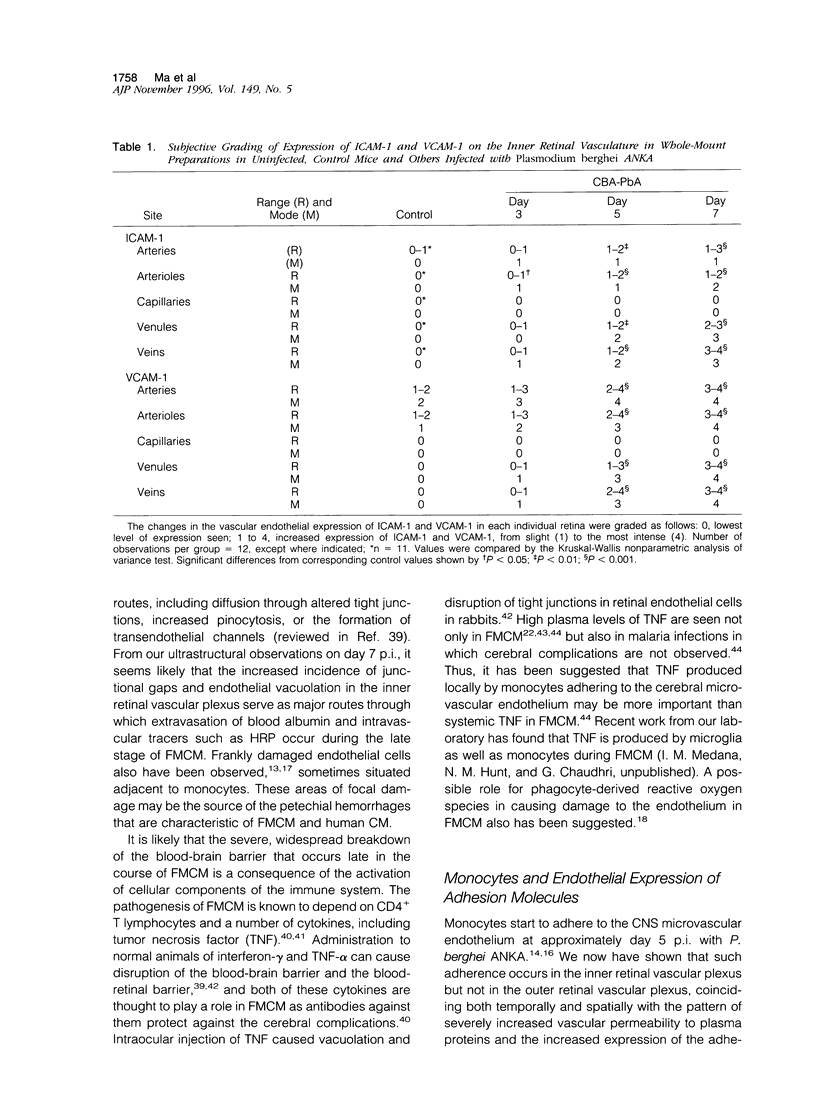
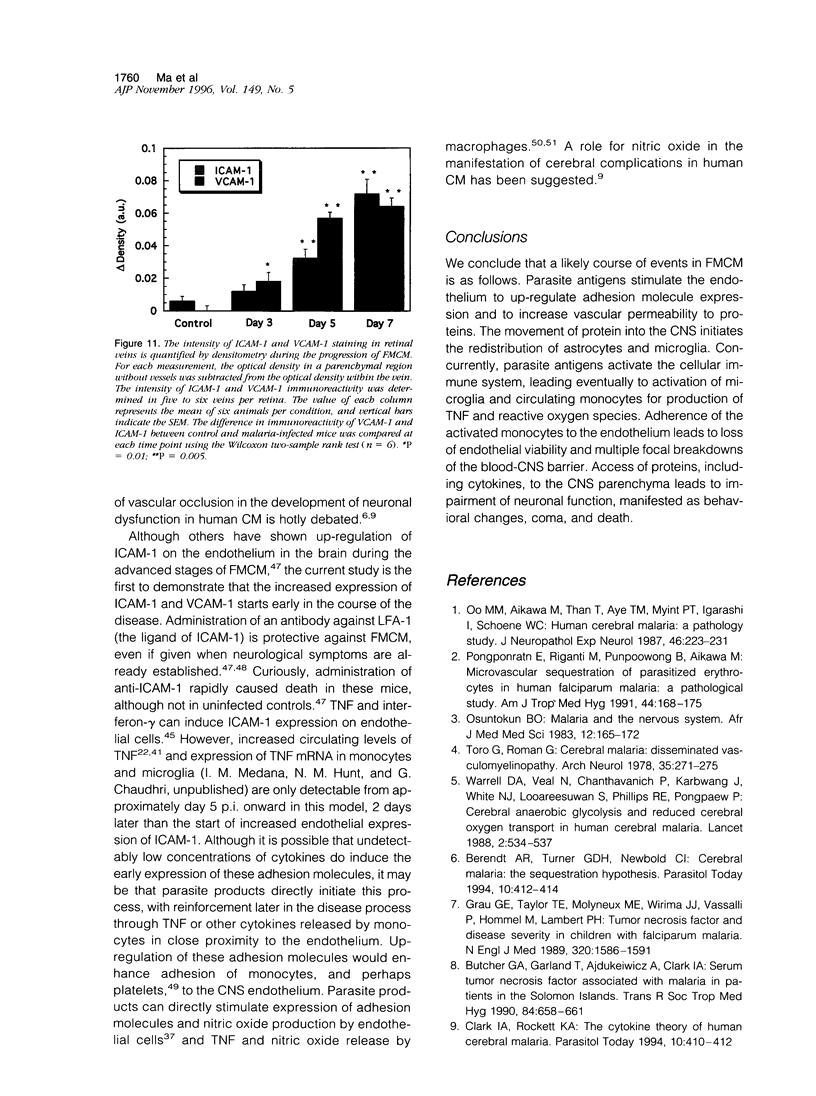
The relationships between increased vascular permeability to protein, monocyte adherence to the endothelium, and expression of the cell adhesion molecules, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in the central nervous system microvasculature were studied during the progression of fatal murine cerebral malaria. CBA mice were inoculated with Plasmodium berghei ANKA, and changes in the retinal microvasculature were examined on days 3, 5, and 7 postinoculation (p.i.). Evans blue dye and horseradish peroxidase (HRP) were administered intravenously to assess vascular permeability to macromolecules macroscopically and by light and electron microscopy. ICAM-1 and VCAM-1 expression were examined by immunohistochemistry. HRP leakage into the retinal parenchyma was seen macroscopically at a low level on day 3 p.i., increasing progressively at day 5 (the earliest time at which cerebral symptoms were observed) and day 7 (the day on which animals showed severe behavioral abnormalities and died). The inner retinal vascular plexus showed a slight increase in vascular permeability to intravenous Evans blue at day 3 p.i. and congestion, monocyte adherence to the endothelium, and increased vascular permeability to both Evans blue and HRP at day 7 p.i. Electron microscopic observations were consistent with these findings and also revealed disrupted light junctions and the coating of monocytes and endothelium with HRP at day 7 p.i. Immunohistochemical staining and densitometry showed a progressive increase from day 3 to day 7 p.i. in the densities of ICAM-1 and VCAM-1 on the venular endothelium of the inner retinal vascular plexus, with the appearance of adherent ICAM-1+ monocytes at the terminal stage of the disease. None of the pathological changes associated with the inner retinal plexus were seen at any stage in the outer retinal plexus. These results suggest the following sequence of events in the inner retinal vessels, particularly the venules, during the progression of fatal murine cerebral malaria: 1) a mild increase in vascular permeability at approximately day 3 p.i., 2) a progressive increase in endothelial expression of the cell adhesion molecules ICAM-1 and VCAM-1, commencing at approximately day 3 p.i., 3) monocyte adhesion to the endothelium starting at approximately day 5 p.i., and 4) frank disruption of endothelial integrity at the terminal stage (day 7 p.i.), leading to edema and hemorrhage. Similar changes in cerebral vessels may underlie the neurological complications of the disease.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Berendt A. R., Tumer G. D., Newbold C. I. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994 Oct;10(10):412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Butcher G. A., Garland T., Ajdukiewicz A. B., Clark I. A. Serum tumor necrosis factor associated with malaria in patients in the Solomon Islands. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):658–661. doi: 10.1016/0035-9203(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T., Tout S., Holländer H., Stone J. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2128–2147. [PubMed] [Google Scholar]
- Chang-Ling T., Neill A. L., Hunt N. H. Early microvascular changes in murine cerebral malaria detected in retinal wholemounts. Am J Pathol. 1992 May;140(5):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Ilschner S., MacMicking J. D., Cowden W. B. TNF and Plasmodium berghei ANKA-induced cerebral malaria. Immunol Lett. 1990 Aug;25(1-3):195–198. doi: 10.1016/0165-2478(90)90114-6. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Rockett K. A. The cytokine theory of human cerebral malaria. Parasitol Today. 1994 Oct;10(10):410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Claudio L., Martiney J. A., Brosnan C. F. Ultrastructural studies of the blood-retina barrier after exposure to interleukin-1 beta or tumor necrosis factor-alpha. Lab Invest. 1994 Jun;70(6):850–861. [PubMed] [Google Scholar]
- Fabry Z., Waldschmidt M. M., Hendrickson D., Keiner J., Love-Homan L., Takei F., Hart M. N. Adhesion molecules on murine brain microvascular endothelial cells: expression and regulation of ICAM-1 and Lgp 55. J Neuroimmunol. 1992 Jan;36(1):1–11. doi: 10.1016/0165-5728(92)90026-h. [DOI] [PubMed] [Google Scholar]
- Falanga P. B., Butcher E. C. Late treatment with anti-LFA-1 (CD11a) antibody prevents cerebral malaria in a mouse model. Eur J Immunol. 1991 Sep;21(9):2259–2263. doi: 10.1002/eji.1830210938. [DOI] [PubMed] [Google Scholar]
- Ghigo D., Todde R., Ginsburg H., Costamagna C., Gautret P., Bussolino F., Ulliers D., Giribaldi G., Deharo E., Gabrielli G. Erythrocyte stages of Plasmodium falciparum exhibit a high nitric oxide synthase (NOS) activity and release an NOS-inducing soluble factor. J Exp Med. 1995 Sep 1;182(3):677–688. doi: 10.1084/jem.182.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Vassalli P., Lambert P. H. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989 Dec;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Pointaire P., Piguet P. F., Vesin C., Rosen H., Stamenkovic I., Takei F., Vassalli P. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol. 1991 Sep;21(9):2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Tacchini-Cottier F., Vesin C., Milon G., Lou J. N., Piguet P. F., Juillard P. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cytokine Netw. 1993 Nov-Dec;4(6):415–419. [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Grau G. E., de Kossodo S. Cerebral malaria: mediators, mechanical obstruction or more? Parasitol Today. 1994 Oct;10(10):408–409. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Greenwood J. Mechanisms of blood-brain barrier breakdown. Neuroradiology. 1991;33(2):95–100. doi: 10.1007/BF00588242. [DOI] [PubMed] [Google Scholar]
- Hidayat A. A., Nalbandian R. M., Sammons D. W., Fleischman J. A., Johnson T. E. The diagnostic histopathologic features of ocular malaria. Ophthalmology. 1993 Aug;100(8):1183–1186. doi: 10.1016/s0161-6420(93)31508-3. [DOI] [PubMed] [Google Scholar]
- Holländer H., Makarov F., Dreher Z., van Driel D., Chan-Ling T. L., Stone J. Structure of the macroglia of the retina: sharing and division of labour between astrocytes and Müller cells. J Comp Neurol. 1991 Nov 22;313(4):587–603. doi: 10.1002/cne.903130405. [DOI] [PubMed] [Google Scholar]
- Hunt N. H., Manduci N., Thumwood C. M. Amelioration of murine cerebral malaria by dietary restriction. Parasitology. 1993 Dec;107(Pt 5):471–476. doi: 10.1017/s0031182000068049. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Morris-Jones S., Rønn A., Hviid L., Theander T. G., Elhassan I. M., Bygbjerg I. C., Greenwood B. M. Increased plasma concentrations of sICAM-1, sVCAM-1 and sELAM-1 in patients with Plasmodium falciparum or P. vivax malaria and association with disease severity. Immunology. 1994 Dec;83(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Lewallen S., Taylor T. E., Molyneux M. E., Wills B. A., Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993 Jun;100(6):857–861. doi: 10.1016/s0161-6420(93)31563-0. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Warrell D. A., White N. J., Chanthavanich P., Warrell M. J., Chantaratherakitti S., Changswek S., Chongmankongcheep L., Kanchanaranya C. Retinal hemorrhage, a common sign of prognostic significance in cerebral malaria. Am J Trop Med Hyg. 1983 Sep;32(5):911–915. doi: 10.4269/ajtmh.1983.32.911. [DOI] [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- McKinney M. M., Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987 Feb 11;96(2):271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- Medana I. M., Chan-Ling T., Hunt N. H. Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier. Glia. 1996 Jan;16(1):51–64. doi: 10.1002/(SICI)1098-1136(199601)16:1<51::AID-GLIA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Chan-Ling T., Hunt N. H. Comparisons between microvascular changes in cerebral and non-cerebral malaria in mice, using the retinal whole-mount technique. Parasitology. 1993 Dec;107(Pt 5):477–487. doi: 10.1017/s0031182000068050. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Hunt N. H. Effects of endotoxin and dexamethasone on cerebral malaria in mice. Parasitology. 1995 Nov;111(Pt 4):443–454. doi: 10.1017/s003118200006594x. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Hunt N. H. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992 Oct;105(Pt 2):165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Ho M., Tandon N. N., Van Seventer G. A., Shaw S., White N. J., Jamieson G. A., Chulay J. D., Webster H. K. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991 Jul;164(1):163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tegoshi T., Maeno Y., Benjamin C., Ho M., Kan K. E., Thway Y., Win K., Aikawa M., Lobb R. R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992 Oct 1;176(4):1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo M. M., Aikawa M., Than T., Aye T. M., Myint P. T., Igarashi I., Schoene W. C. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987 Mar;46(2):223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Osuntokun B. O. Malaria and the nervous system. Afr J Med Med Sci. 1983 Sep-Dec;12(3-4):165–172. [PubMed] [Google Scholar]
- Patnaik J. K., Das B. S., Mishra S. K., Mohanty S., Satpathy S. K., Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994 Nov;51(5):642–647. [PubMed] [Google Scholar]
- Pongponratn E., Riganti M., Punpoowong B., Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991 Feb;44(2):168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- Prieto J., Takei F., Gendelman R., Christenson B., Biberfeld P., Patarroyo M. MALA-2, mouse homologue of human adhesion molecule ICAM-1 (CD54). Eur J Immunol. 1989 Sep;19(9):1551–1557. doi: 10.1002/eji.1830190906. [DOI] [PubMed] [Google Scholar]
- Rest J. R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76(3):410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Tuor U. I. Blood-eye barriers in the rat: correlation of ultrastructure with function. J Comp Neurol. 1994 Feb 22;340(4):566–576. doi: 10.1002/cne.903400409. [DOI] [PubMed] [Google Scholar]
- Tachado S. D., Schofield L. Glycosylphosphatidylinositol toxin of Trypanosoma brucei regulates IL-1 alpha and TNF-alpha expression in macrophages by protein tyrosine kinase mediated signal transduction. Biochem Biophys Res Commun. 1994 Dec 15;205(2):984–991. doi: 10.1006/bbrc.1994.2763. [DOI] [PubMed] [Google Scholar]
- Thumwood C. M., Hunt N. H., Clark I. A., Cowden W. B. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology. 1988 Jun;96(Pt 3):579–589. doi: 10.1017/s0031182000080203. [DOI] [PubMed] [Google Scholar]
- Thumwood C. M., Hunt N. H., Cowden W. B., Clark I. A. Antioxidants can prevent cerebral malaria in Plasmodium berghei-infected mice. Br J Exp Pathol. 1989 Jun;70(3):293–303. [PMC free article] [PubMed] [Google Scholar]
- Toro G., Román G. Cerebral malaria. A disseminated vasculomyelinopathy. Arch Neurol. 1978 May;35(5):271–275. doi: 10.1001/archneur.1978.00500290017004. [DOI] [PubMed] [Google Scholar]
- Turner G. D., Morrison H., Jones M., Davis T. M., Looareesuwan S., Buley I. D., Gatter K. C., Newbold C. I., Pukritayakamee S., Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994 Nov;145(5):1057–1069. [PMC free article] [PubMed] [Google Scholar]
- Warrell D. A., White N. J., Veall N., Looareesuwan S., Chanthavanich P., Phillips R. E., Karbwang J., Pongpaew P., Krishna S. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet. 1988 Sep 3;2(8610):534–538. doi: 10.1016/s0140-6736(88)92658-x. [DOI] [PubMed] [Google Scholar]
- Wenisch C., Varijanonta S., Looareesuwan S., Graninger W., Pichler R., Wernsdorfer W. Soluble intercellular adhesion molecule-1 (ICAM-1), endothelial leukocyte adhesion molecule-1 (ELAM-1), and tumor necrosis factor receptor (55 kDa TNF-R) in patients with acute Plasmodium falciparum malaria. Clin Immunol Immunopathol. 1994 Jun;71(3):344–348. doi: 10.1006/clin.1994.1096. [DOI] [PubMed] [Google Scholar]
- de Kossodo S., Grau G. E. Role of cytokines and adhesion molecules in malaria immunopathology. Stem Cells. 1993 Jan;11(1):41–48. doi: 10.1002/stem.5530110108. [DOI] [PubMed] [Google Scholar]