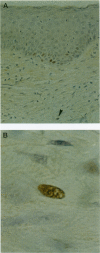
Abstract
Keloids are collagenous lesions acquired as a result of abnormal wound heating. In this study we have assessed the potential role of proliferation, apoptosis, and necrosis in keloids. Samples were immunolabeled for proliferating cell nuclear antigen or DNA strand breaks or stained with acridine orange. Proliferating cells were observed in the basal layer of the epidermis and fibroblasts in the dermis, the numbers of the latter being increased in comparison with normal skin. No proliferating cells were observed in the central region of the keloid. In normal skin, apoptotic cells were restricted to the basal layer of the epidermis. In keloid samples, numerous apoptotic cells were observed in the epidermis and dermis; the number and distribution of positive cells decreased more distal to the keloid lesion. Apoptotic endothelial cells of a small proportion of blood vessels in the dermis were also observed. Evidence of necrosis was also seen in the dermis. These results suggest that, with maturity, progressive cell degeneration primarily by apoptosis results in clearance of certain cellular populations resulting in the typical keloid lesion. However, the persistence of fibroblast proliferation at the dermal/keloid interface propagates the fibrosis.
Full text
PDF

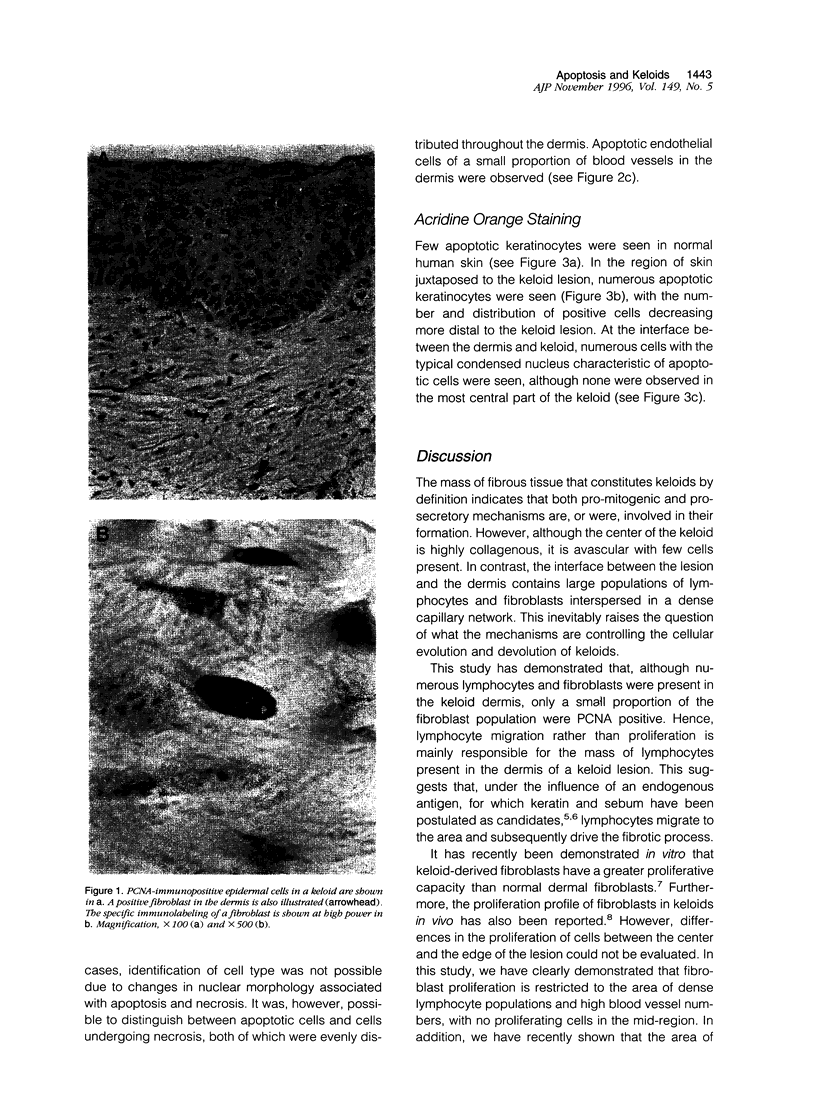
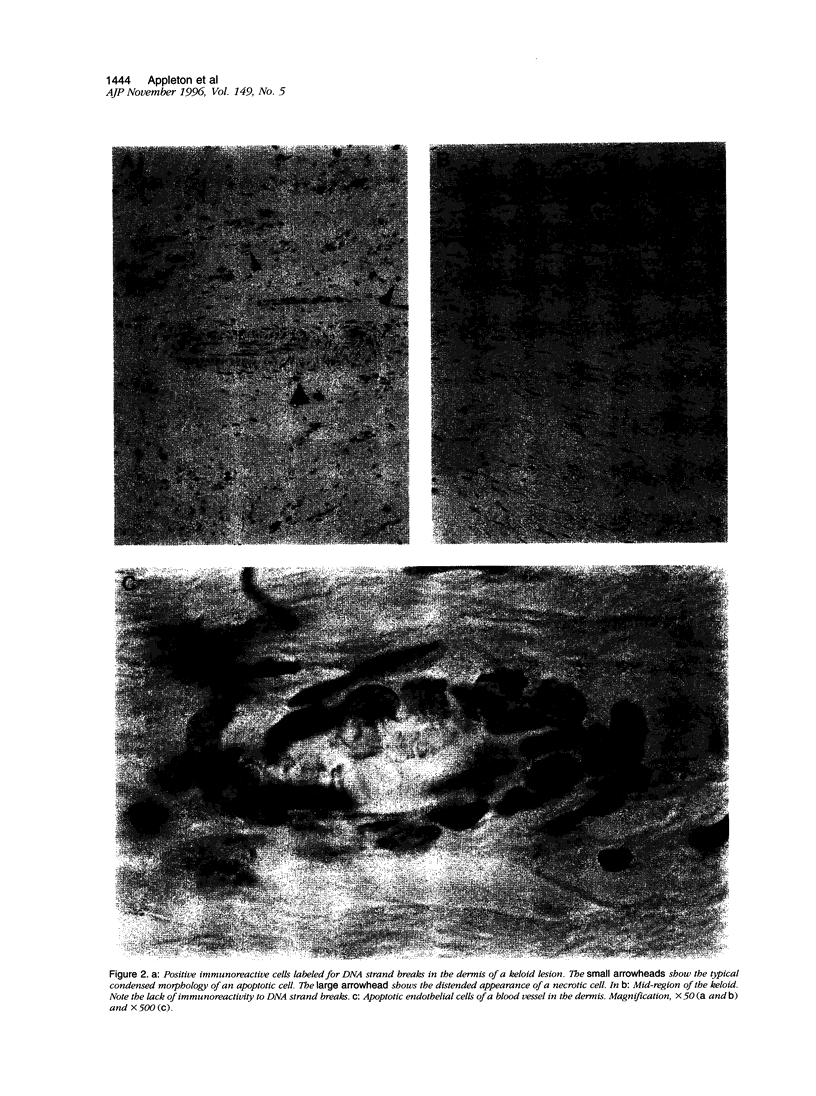
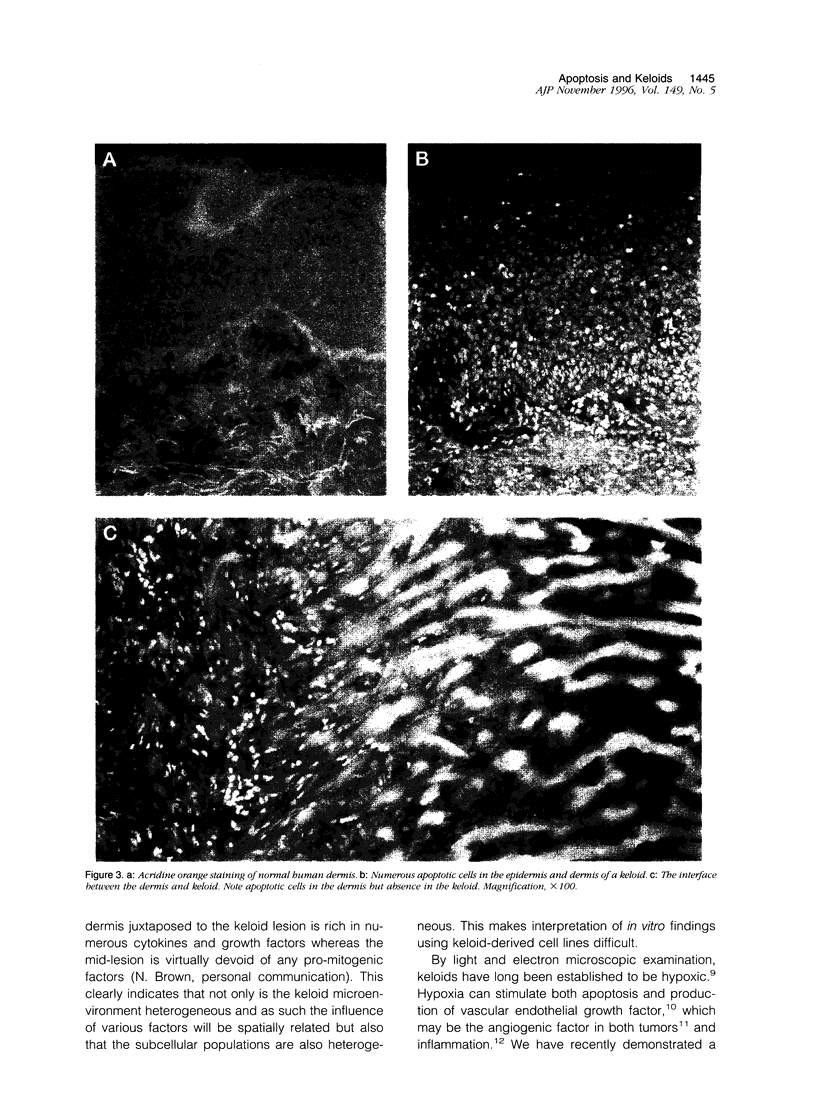


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhady S. M., Sivanantharajah K. Keloids in various races. A review of 175 cases. Plast Reconstr Surg. 1969 Dec;44(6):564–566. doi: 10.1097/00006534-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Ansari B., Coates P. J., Greenstein B. D., Hall P. A. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993 May;170(1):1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- Calderon M., Lawrence W. T., Banes A. J. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res. 1996 Mar;61(2):343–347. doi: 10.1006/jsre.1996.0127. [DOI] [PubMed] [Google Scholar]
- Cohen I. K., McCoy B. J., Mohanakumar T., Diegelmann R. F. Immunoglobulin, complement, and histocompatibility antigen studies in keloid patients. Plast Reconstr Surg. 1979 May;63(5):689–695. doi: 10.1097/00006534-197905000-00013. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Don M. M., Ablett G., Bishop C. J., Bundesen P. G., Donald K. J., Searle J., Kerr J. F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust J Exp Biol Med Sci. 1977 Aug;55(4):407–417. doi: 10.1038/icb.1977.38. [DOI] [PubMed] [Google Scholar]
- Grubauer G., Romani N., Kofler H., Stanzl U., Fritsch P., Hintner H. Apoptotic keratin bodies as autoantigen causing the production of IgM-anti-keratin intermediate filament autoantibodies. J Invest Dermatol. 1986 Oct;87(4):466–471. doi: 10.1111/1523-1747.ep12455510. [DOI] [PubMed] [Google Scholar]
- Kazeem A. A. The immunological aspects of keloid tumor formation. J Surg Oncol. 1988 May;38(1):16–18. doi: 10.1002/jso.2930380106. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischer C. W., Pindur J., Krasovitch P., Kischer E. Characteristics of granulation tissue which promote hypertrophic scarring. Scanning Microsc. 1990 Dec;4(4):877–888. [PubMed] [Google Scholar]
- Kischer C. W. The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol. 1992 Apr;24(2):281–296. [PubMed] [Google Scholar]
- Klagsbrun M., Soker S. VEGF/VPF: the angiogenesis factor found? Curr Biol. 1993 Oct 1;3(10):699–702. doi: 10.1016/0960-9822(93)90073-w. [DOI] [PubMed] [Google Scholar]
- MOWLEM R. Hypertrophic scars. Br J Plast Surg. 1951 Jul;4(2):113–120. doi: 10.1016/s0007-1226(51)80016-x. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Pollack S. V., Pinnell S. R. Keloids: a review. J Am Acad Dermatol. 1981 Apr;4(4):461–470. doi: 10.1016/s0190-9622(81)70048-3. [DOI] [PubMed] [Google Scholar]
- Nakaoka H., Miyauchi S., Miki Y. Proliferating activity of dermal fibroblasts in keloids and hypertrophic scars. Acta Derm Venereol. 1995 Mar;75(2):102–104. doi: 10.2340/0001555575102104. [DOI] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Battersby C., Egerton W. S., Balderson G., Burnett W. An electron microscopic study of the mode of donor cell death in unmodified rejection of pig liver allografts. Aust J Exp Biol Med Sci. 1977 Aug;55(4):401–406. doi: 10.1038/icb.1977.37. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Yagi K. I., Dafalla A. A., Osman A. A. Does an immune reaction to sebum in wounds cause keloid scars? Beneficial effect of desensitisation. Br J Plast Surg. 1979 Jul;32(3):223–225. doi: 10.1016/s0007-1226(79)90037-7. [DOI] [PubMed] [Google Scholar]