Abstract
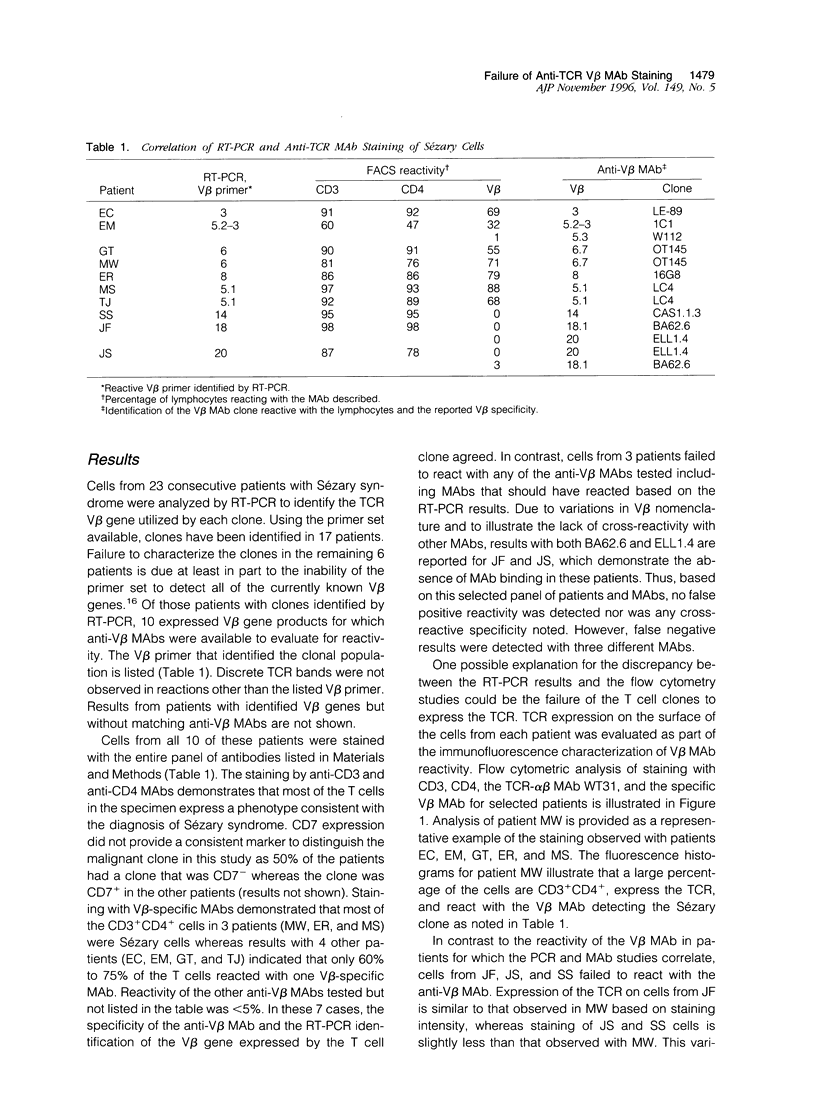
Monoclonal antibodies (MAbs) reacting with the human T cell receptor (TCR) V beta or V alpha region have been shown to be almost as specific as a private idiotypic MAb in identifying T cell clones. When available, V beta-specific MAbs offer the ease of immunofluorescence analysis to identify and quantitate expanded malignant or nonmalignant T cell populations without requiring polymerase chain reaction (PCR) technology to evaluate expression of V beta gene families. The V beta expression of peripheral blood lymphocytes from twenty-three consecutive patients with Sézary syndrome has been analyzed by reverse transcriptase (RT)-PCR. Ten patients had malignant T cell clones that expressed a TCR V beta corresponding to a commercially available anti-V beta antibody. Immunofluorescence staining with anti-V beta MAbs showed a direct correlation with RT-PCR results in seven of ten patients. No false positive reactivity was noted on immunofluorescence staining with any MAb. Cells from three patients, however, did not react with the corresponding anti-V beta MAb. These three cases expressed a TCR V beta from gene families containing a single member, ie, V beta 14, V beta 18, and V beta 20, yet MAbs reported to be specific for these regions failed to react with the T cell clone from these patients. Sequencing of the PCR product in these cases confirmed the RT-PCR results. Cells from two patients expressed a TCR using V beta 5.1-D beta 1.1 genes with different J-C segments. One patient's cells reacted with an anti-V beta 5.1 MAb (LC4) whereas the other patient's cells bound one-tenth the amount of this same MAb. These results indicate that currently available anti-TCR V region MAbs may not react consistently with T cell clones expressing the corresponding V region or may react with a low affinity making detection difficult. Differences in the J-C junction or in CDR3 may influence the binding of these MAbs. Until the false negative rate is reduced and the fine specificity and affinity of these MAbs is better characterized, both PCR and MAb studies will be required to reliably identify and quantitate clonal T cell populations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahler D. W., Berry G., Oksenberg J., Warnke R. A., Levy R. Diversity of T-cell antigen receptor variable genes used by mycosis fungoides cells. Am J Pathol. 1992 Jan;140(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Bigler R. D., Fisher D. E., Wang C. Y., Rinnooy Kan E. A., Kunkel H. G. Idiotype-like molecules on cells of a human T cell leukemia. J Exp Med. 1983 Sep 1;158(3):1000–1005. doi: 10.1084/jem.158.3.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley M., McCoy J. P., Deng J. S., Jegasothy B. Anti-V region antibodies as "almost clonotypic" reagents for the study of cutaneous T cell lymphomas and leukemias. J Invest Dermatol. 1990 Nov;95(5):614–617. doi: 10.1111/1523-1747.ep12505618. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian H., Sugita M., Glass D. N., Maier A. L., Weinblatt M. E., Rème T., Brenner M. B. Clonal V alpha 12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med. 1993 Jun 1;177(6):1623–1631. doi: 10.1084/jem.177.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diu A., Romagné F., Genevée C., Rocher C., Bruneau J. M., David A., Praz F., Hercend T. Fine specificity of monoclonal antibodies directed at human T cell receptor variable regions: comparison with oligonucleotide-driven amplification for evaluation of V beta expression. Eur J Immunol. 1993 Jul;23(7):1422–1429. doi: 10.1002/eji.1830230703. [DOI] [PubMed] [Google Scholar]
- Fivenson D. P., Nickoloff B. J. Immunodiagnosis in cutaneous T cell lymphoma: how does gene expression of the variable region of the T cell receptor fit into the diagnostic and pathophysiological picture of T cell neoplasia. J Cutan Pathol. 1992 Feb;19(1):1–5. doi: 10.1111/j.1600-0560.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Gorochov G., Bachelez H., Cayuela J. M., Legac E., Laroche L., Dubertret L., Sigaux F. Expression of V beta gene segments by Sezary cells. J Invest Dermatol. 1995 Jul;105(1):56–61. doi: 10.1111/1523-1747.ep12312560. [DOI] [PubMed] [Google Scholar]
- Gorski J., Yassai M., Zhu X., Kissela B., Kissella B [corrected to Kissela B. ]., Keever C., Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994 May 15;152(10):5109–5119. [PubMed] [Google Scholar]
- Heber-Katz E., Acha-Orbea H. The V-region disease hypothesis: evidence from autoimmune encephalomyelitis. Immunol Today. 1989 May;10(5):164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- Janson C. H., Tehrani M. J., Mellstedt H., Wigzell H. Anti-idiotypic monoclonal antibody to a T-cell chronic lymphatic leukemia. Characterization of the antibody, in vitro effector functions and results of therapy. Cancer Immunol Immunother. 1989;28(3):225–232. doi: 10.1007/BF00204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Leung D. Y., Kappler J., Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- Kumar V., Kono D. H., Urban J. L., Hood L. The T-cell receptor repertoire and autoimmune diseases. Annu Rev Immunol. 1989;7:657–682. doi: 10.1146/annurev.iy.07.040189.003301. [DOI] [PubMed] [Google Scholar]
- Lessin S. R., Rook A. H., Rovera G. Molecular diagnosis of cutaneous T-cell lymphoma: polymerase chain reaction amplification of T-cell antigen receptor beta-chain gene rearrangements. J Invest Dermatol. 1991 Mar;96(3):299–302. doi: 10.1111/1523-1747.ep12465108. [DOI] [PubMed] [Google Scholar]
- Poppema S., Hepperle B. Restricted V gene usage in T-cell lymphomas as detected by anti-T-cell receptor variable region reagents. Am J Pathol. 1991 Jun;138(6):1479–1484. [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., Bigler R. D., Bushkin Y., Fisher D. E., Wang C. Y., Mayer L. F., Chiorazzi N., Kunkel H. G. T cell antiidiotypic antibodies reveal differences between two human leukemias. J Exp Med. 1984 Aug 1;160(2):494–505. doi: 10.1084/jem.160.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preesman A. H., Hu H. Z., Tilanus M. G., de Geus B., Schuurman H. J., Reitsma R., van Wichen D. F., van Vloten W. A., de Weger R. A. T-cell receptor V beta-family usage in primary cutaneous and primary nodal T-cell non-Hodgkin's lymphomas. J Invest Dermatol. 1992 Nov;99(5):587–593. doi: 10.1111/1523-1747.ep12667988. [DOI] [PubMed] [Google Scholar]
- Romagné F., Besnardeau L., Malissen B. A versatile method to produce antibodies to human T cell receptor V beta segments: frequency determination of human V beta 2+ T cells that react with toxic-shock syndrome toxin-1. Eur J Immunol. 1992 Oct;22(10):2749–2752. doi: 10.1002/eji.1830221043. [DOI] [PubMed] [Google Scholar]
- Wei S., Charmley P., Robinson M. A., Concannon P. The extent of the human germline T-cell receptor V beta gene segment repertoire. Immunogenetics. 1994;40(1):27–36. doi: 10.1007/BF00163961. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Hu E., Wood G. S., Moulds C., Cleary M. L., Warnke R., Sklar J. Clonal rearrangements of T-cell receptor genes in mycosis fungoides and dermatopathic lymphadenopathy. N Engl J Med. 1985 Aug 29;313(9):539–544. doi: 10.1056/NEJM198508293130903. [DOI] [PubMed] [Google Scholar]


