Abstract
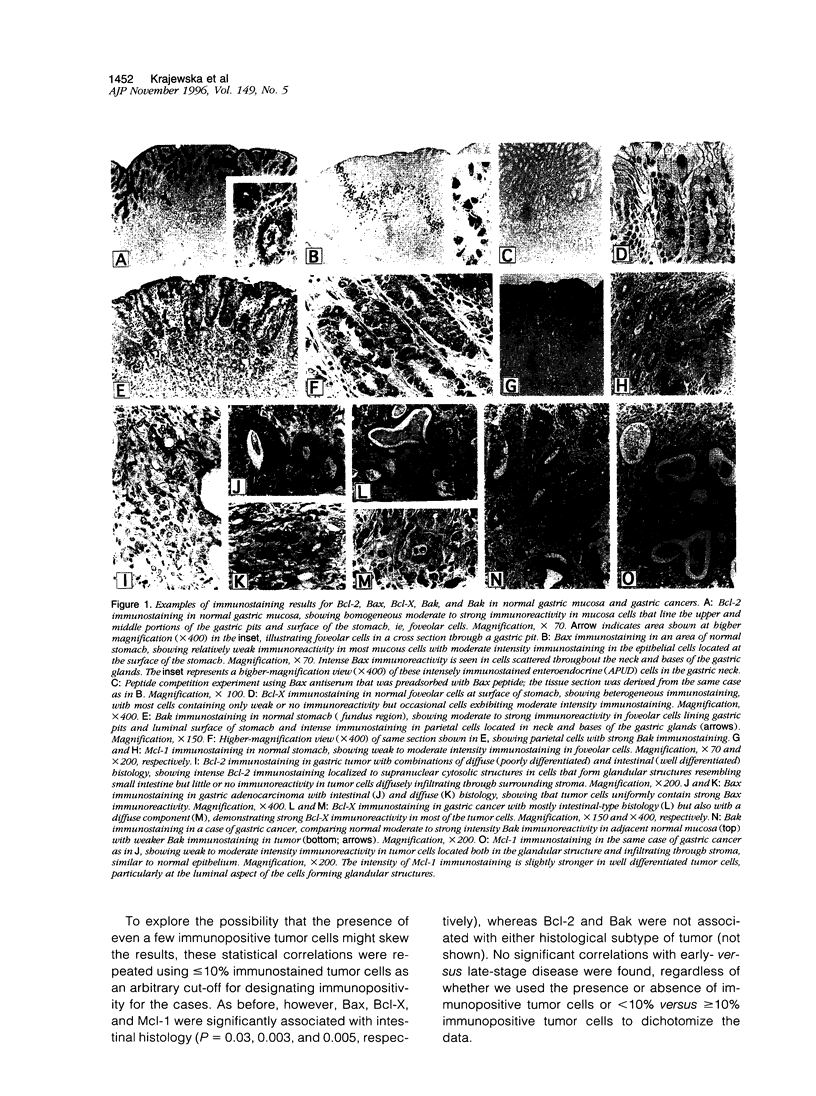
The apoptosis-regulating proteins Bcl-2, Bax, Bcl-X, Bak, and Mcl-1 were examined by immunohistochemical methods in 48 archival specimens of adenocarcinoma of the stomach, and the results were correlated with tumor histology (intestinal versus diffuse pattern) and clinical stage (early- versus late-stage disease, ie, stages I and II versus stage III). Tumor cells containing immunostaining for the anti-apoptotic proteins Bcl-2, Bcl-X, and Mcl-1 were present in 26 (54%), 41 (85%), and 36 (75%) of the 48 cases evaluated, respectively, whereas immunopositivity for the pro-apoptotic proteins Bax and Bak was found in 44 (92%) and 42 (88%) specimens Comparisons of these immunostaining results with tumor histology revealed statistically significant differences for Bax (P = 0.03), Bcl-X (P = 0.003), and Mcl-1 (P = 0.005), which were all more frequently immunopositive for tumors with an intestinal than a diffuse histological pattern (chi 2 analysis). In addition, the percentage of immunopositive tumor cells was significantly higher for Bcl-X (62 +/- 6% versus 45 +/- 6%, mean +/- SE, P = 0.01) and for Mcl-1 (48 +/- 6% versus 30 +/- 6%; P = 0.04) in tumors with intestinal versus diffuse histology (unpaired t-test). In contrast, the percentage of Bcl-2-immunopositive tumor cells was higher in tumors with diffuse histology compared with intestinal (32 +/- 5% versus 12 +/- 5%; P = 0.01), whereas the percentages of Bax- and Bak-immunopositive tumor cells were not significantly different between these two histological types. In 34 specimens, residual normal gastric epithelial cells (foveolar cells) were present for direct comparisons of immunointensity with tumor cells. The immunointensity for the Bcl-2, Bcl-X, and Mcl-1 proteins was stronger in tumor cells compared with normal foveolar cells in 7 (21%), 15 (44%), and 8 (2.1%) of 34 cases, respectively, whereas the immunointensity of the proapoptotic proteins Bax and Bak was reduced compared with normal cells in 8 (24%) and 24 (71%) cases. Immunointensity, however, did not correlate with histology. clinical stage was not significantly associated with the presence or absence of immunopositive tumor cells, the percentage of immunopositive cells, or immunointensity. Taken together, these results establish for the first time that several Bcl-2 family proteins are expressed in gastric adenocarcinomas and suggest that the repertoire of these proteins may differ depending on the histological type. The findings therefore support the notion that the intestinal and diffuse types of gastric cancer arise at least in part through different mechanisms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayhan A., Yasui W., Yokozaki H., Seto M., Ueda R., Tahara E. Loss of heterozygosity at the bcl-2 gene locus and expression of bcl-2 in human gastric and colorectal carcinomas. Jpn J Cancer Res. 1994 Jun;85(6):584–591. doi: 10.1111/j.1349-7006.1994.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou R. C., Daniel P. T., Mapara M. Y., Bommert K., Wagener C., Kallinich B., Royer H. D., Dörken B. Expression of the bcl-2 gene family in normal and malignant breast tissue: low bax-alpha expression in tumor cells correlates with resistance towards apoptosis. Int J Cancer. 1995 Mar 16;60(6):854–859. doi: 10.1002/ijc.2910600622. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Broome H. E., Dargan C. M., Krajewski S., Reed J. C. Expression of Bcl-2, Bcl-x, and Bax after T cell activation and IL-2 withdrawal. J Immunol. 1995 Sep 1;155(5):2311–2317. [PubMed] [Google Scholar]
- Chittenden T., Harrington E. A., O'Connor R., Flemington C., Lutz R. J., Evan G. I., Guild B. C. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- Farrow S. N., White J. H., Martinou I., Raven T., Pun K. T., Grinham C. J., Martinou J. C., Brown R. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995 Apr 20;374(6524):731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- Furuya Y., Krajewski S., Epstein J. I., Reed J. C., Isaacs J. T. Expression of bcl-2 and the progression of human and rodent prostatic cancers. Clin Cancer Res. 1996 Feb;2(2):389–398. [PubMed] [Google Scholar]
- Hahn S. A., Schutte M., Hoque A. T., Moskaluk C. A., da Costa L. T., Rozenblum E., Weinstein C. L., Fischer A., Yeo C. J., Hruban R. H. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996 Jan 19;271(5247):350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Brauer M. J., Powers V. C., Wu J. J., Umansky S. R., Tomei L. D., Barr P. J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Knudson C. M., Tung K. S., Tourtellotte W. G., Brown G. A., Korsmeyer S. J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995 Oct 6;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Krajewska M., Krajewski S., Epstein J. I., Shabaik A., Sauvageot J., Song K., Kitada S., Reed J. C. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996 May;148(5):1567–1576. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Blomqvist C., Franssila K., Krajewska M., Wasenius V. M., Niskanen E., Nordling S., Reed J. C. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995 Oct 1;55(19):4471–4478. [PubMed] [Google Scholar]
- Krajewski S., Bodrug S., Gascoyne R., Berean K., Krajewska M., Reed J. C. Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol. 1994 Sep;145(3):515–525. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Bodrug S., Krajewska M., Shabaik A., Gascoyne R., Berean K., Reed J. C. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol. 1995 Jun;146(6):1309–1319. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Chatten J., Hanada M., Reed J. C. Immunohistochemical analysis of the Bcl-2 oncoprotein in human neuroblastomas. Comparisons with tumor cell differentiation and N-Myc protein. Lab Invest. 1995 Jan;72(1):42–54. [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Reed J. C. Immunohistochemical analysis of in vivo patterns of Bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Res. 1996 Jun 15;56(12):2849–2855. [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Miyashita T., Wang H. G., Reed J. C. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994 Dec;145(6):1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Wang H. G., Irie S., Fong L., Reed J. C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994 Nov 1;54(21):5501–5507. [PubMed] [Google Scholar]
- Krajewski S., Mai J. K., Krajewska M., Sikorska M., Mossakowski M. J., Reed J. C. Upregulation of bax protein levels in neurons following cerebral ischemia. J Neurosci. 1995 Oct;15(10):6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2: prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995 Apr;9(2):451–473. [PubMed] [Google Scholar]
- Reynolds J. E., Yang T., Qian L., Jenkinson J. D., Zhou P., Eastman A., Craig R. W. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994 Dec 15;54(24):6348–6352. [PubMed] [Google Scholar]
- Ruoslahti E., Reed J. C. Anchorage dependence, integrins, and apoptosis. Cell. 1994 May 20;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Tahara E. Molecular biology of gastric cancer. World J Surg. 1995 Jul-Aug;19(4):484–490. doi: 10.1007/BF00294705. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Wingo P. A., Tong T., Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995 Jan-Feb;45(1):8–30. doi: 10.3322/canjclin.45.1.8. [DOI] [PubMed] [Google Scholar]



