Abstract
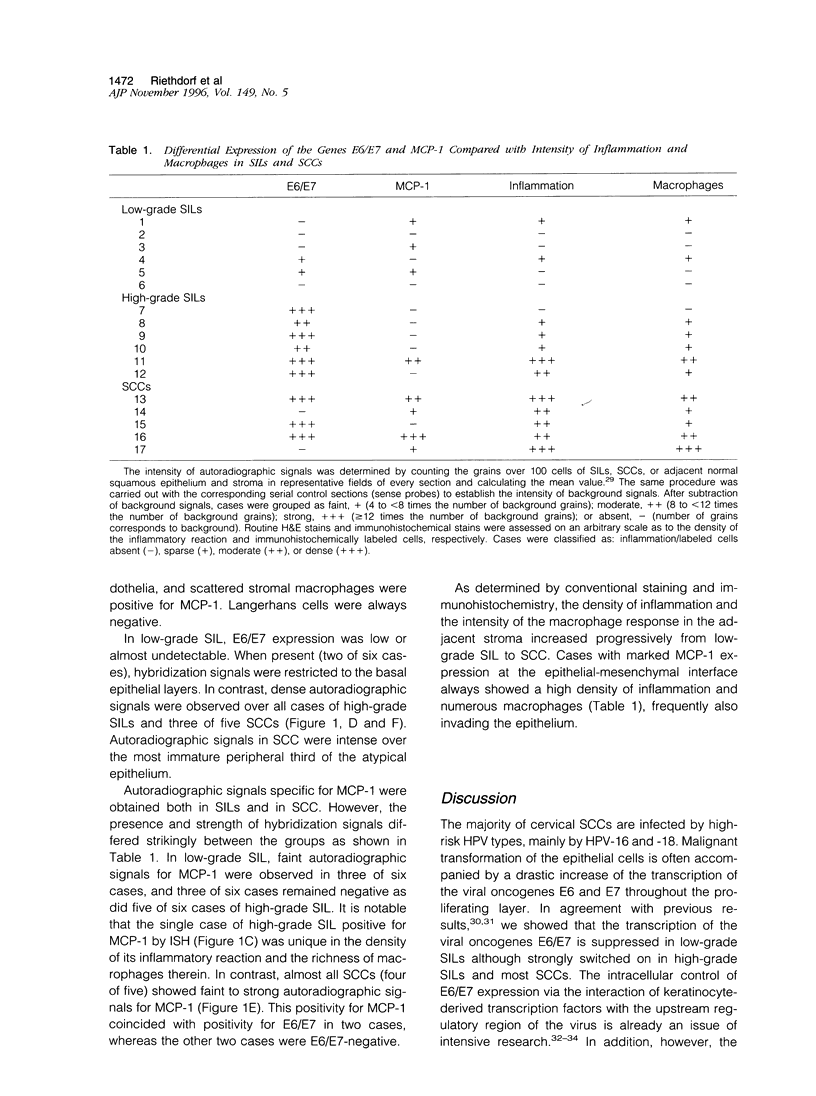
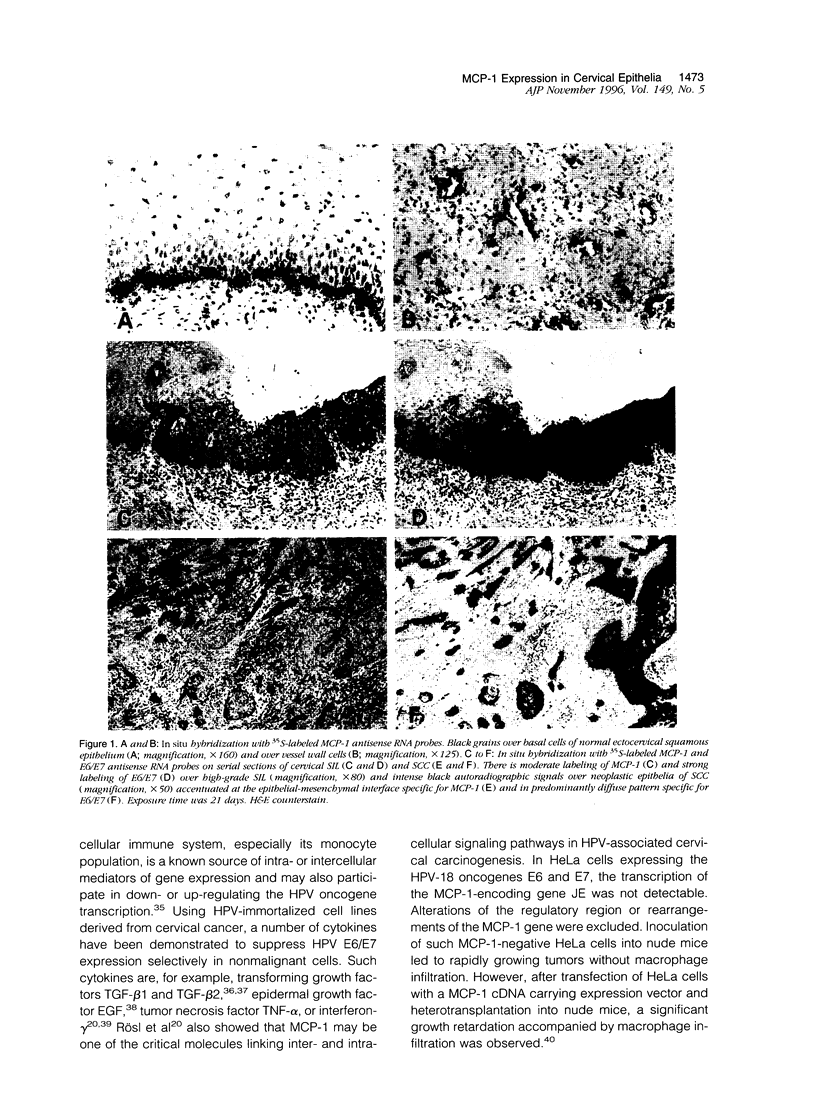
Monocyte chemoattractant protein (MCP)-1 is an important factor involved in the cross-talk between mononuclear cells and human papilloma-virus (HPV)-infected cervical epithelia. To prove the experimental model of a negative regulatory loop between the expression of the HPV oncogenes E6/E7 and the MCP-1 gene in vivo, we examined HPV-16-infected conization/hysterectomy specimens consisting of 6 low- and 6 high-grade squamous intraepithelial lesions (SILs) and 5 squamous cell carcinomas (SCCs) as well as the adjacent mucosa by isotopic RNA in situ hybridization. Langerhans cells and stromal macrophages were identified by the antibodies S-100 and PG-M1. E6/E7 expression was restricted to dysplastic or neoplastic keratinocytes, whereas MCP-1 transcripts were detectable in normal, dysplastic, and neoplastic epithelia, in endothelia, and in stromal macrophages. Langerhans cells were always negative. MCP-1 expression was predominant at the epithelial-mesenchymal junctions and especially intense when the stromal macrophage reaction was increased. Generally, the synchronous expression of E6/E7 and MCP-1 was very rare in SILs (2 of 12 cases). In high-grade SIL, MCP-1 expression was observed in 1 of 6 cases, whereas all lesions strongly hybridized with E6/E7 probes. In contrast, 4 of 5 SCCs re-expressed MCP-1, and 2 cases revealed marked transcriptional activities for both E6/E7 and MCP-1. Although preliminary, our observations lend support to the suggestion that the experimental model of transcriptional regulation and exclusion of either HPV E6/E7 or MCP-1 expression is especially pertinent to high-grade SIL, whereas in most SCCs, other environmental factors may influence this relationship.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloub M. I., Barr B. B., McLaren K. M., Smith I. W., Bunney M. H., Smart G. E. Human papillomavirus infection and cervical intraepithelial neoplasia in women with renal allografts. BMJ. 1989 Jan 21;298(6667):153–156. doi: 10.1136/bmj.298.6667.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apt D., Chong T., Liu Y., Bernard H. U. Nuclear factor I and epithelial cell-specific transcription of human papillomavirus type 16. J Virol. 1993 Aug;67(8):4455–4463. doi: 10.1128/jvi.67.8.4455-4463.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L., Moreau F., Vousden K., Pim D., Matlashewski G. Expression of the human papillomavirus E7 oncogene during cell transformation is sufficient to induce susceptibility to lysis by activated macrophages. J Immunol. 1991 Mar 15;146(6):2037–2042. [PubMed] [Google Scholar]
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Braun L., Dürst M., Mikumo R., Gruppuso P. Differential response of nontumorigenic and tumorigenic human papillomavirus type 16-positive epithelial cells to transforming growth factor beta 1. Cancer Res. 1990 Nov 15;50(22):7324–7332. [PubMed] [Google Scholar]
- Chang H. C., Hsu F., Freeman G. J., Griffin J. D., Reinherz E. L. Cloning and expression of a gamma-interferon-inducible gene in monocytes: a new member of a cytokine gene family. Int Immunol. 1989;1(4):388–397. doi: 10.1093/intimm/1.4.388. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Denis M., Chadee K., Matlashewski G. J. Macrophage killing of human papillomavirus type 16-transformed cells. Virology. 1989 May;170(1):342–345. doi: 10.1016/0042-6822(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Dürst M., Glitz D., Schneider A., zur Hausen H. Human papillomavirus type 16 (HPV 16) gene expression and DNA replication in cervical neoplasia: analysis by in situ hybridization. Virology. 1992 Jul;189(1):132–140. doi: 10.1016/0042-6822(92)90688-l. [DOI] [PubMed] [Google Scholar]
- Iglesias M., Plowman G. D., Woodworth C. D. Interleukin-6 and interleukin-6 soluble receptor regulate proliferation of normal, human papillomavirus-immortalized, and carcinoma-derived cervical cells in vitro. Am J Pathol. 1995 Apr;146(4):944–952. [PMC free article] [PubMed] [Google Scholar]
- Ishiji T., Lace M. J., Parkkinen S., Anderson R. D., Haugen T. H., Cripe T. P., Xiao J. H., Davidson I., Chambon P., Turek L. P. Transcriptional enhancer factor (TEF)-1 and its cell-specific co-activator activate human papillomavirus-16 E6 and E7 oncogene transcription in keratinocytes and cervical carcinoma cells. EMBO J. 1992 Jun;11(6):2271–2281. doi: 10.1002/j.1460-2075.1992.tb05286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Allen-Hoffmann B. L., Lambert P. F. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J Virol. 1995 May;69(5):2989–2997. doi: 10.1128/jvi.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Lambert P. F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim S. A., Rees R. C. Enhancement of tumor growth in mice: evidence for the involvement of host macrophages. Cell Immunol. 1984 Aug;87(1):259–269. doi: 10.1016/0008-8749(84)90150-3. [DOI] [PubMed] [Google Scholar]
- Kleine K., König G., Kreuzer J., Komitowski D., Zur Hausen H., Rösl F. The effect of the JE (MCP-1) gene, which encodes monocyte chemoattractant protein-1, on the growth of HeLa cells and derived somatic-cell hybrids in nude mice. Mol Carcinog. 1995 Nov;14(3):179–189. doi: 10.1002/mc.2940140307. [DOI] [PubMed] [Google Scholar]
- Maiman M., Fruchter R. G., Guy L., Cuthill S., Levine P., Serur E. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer. 1993 Jan 15;71(2):402–406. doi: 10.1002/1097-0142(19930115)71:2<402::aid-cncr2820710222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bottazzi B., Colotta F., Sozzani S., Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992 Jul;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest. 1994 Jul;71(1):5–16. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Surrenti C., Riecken E. O., Stein H. Cellular localization of type I III and IV procollagen gene transcripts in normal and fibrotic human liver. Am J Pathol. 1990 Jul;137(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Milde-Langosch K., Becker G., Löning T. Human papillomavirus and c-myc/c-erbB2 in uterine and vulvar lesions. Virchows Arch A Pathol Anat Histopathol. 1991;419(6):479–485. doi: 10.1007/BF01650676. [DOI] [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- Negus R. P., Stamp G. W., Relf M. G., Burke F., Malik S. T., Bernasconi S., Allavena P., Sozzani S., Mantovani A., Balkwill F. R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995 May;95(5):2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Namkoong S. E., Lee H. Y., Kim S. J., Daniel R. W., Shah K. V. Detection of human papillomavirus genotypes in cervical neoplasia from Korean women using polymerase chain reaction. Gynecol Oncol. 1991 May;41(2):129–134. doi: 10.1016/0090-8258(91)90271-6. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Sunday M. E. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol. 1991 Jun;11(6):3125–3131. doi: 10.1128/mcb.11.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösl F., Lengert M., Albrecht J., Kleine K., Zawatzky R., Schraven B., zur Hausen H. Differential regulation of the JE gene encoding the monocyte chemoattractant protein (MCP-1) in cervical carcinoma cells and derived hybrids. J Virol. 1994 Apr;68(4):2142–2150. doi: 10.1128/jvi.68.4.2142-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Sciacca F. L., Stürzl M., Bussolino F., Sironi M., Brandstetter H., Zietz C., Zhou D., Matteucci C., Peri G., Sozzani S. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol. 1994 Nov 15;153(10):4816–4825. [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Rhodes C. R., Whitbeck A., Wolinsky S. M., Chow L. T., Broker T. R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992 Feb;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Standiford T. J., Basha M. A., Showell H. J., Kunkel S. L. Disparate gene expression of chemotactic cytokines by human mononuclear phagocytes. Biochem Biophys Res Commun. 1990 Jan 30;166(2):886–891. doi: 10.1016/0006-291x(90)90893-r. [DOI] [PubMed] [Google Scholar]
- Tagami H., Oguchi M., Ofuji S. The phenomenon of spontaneous regression of numerous flat warts: immunohistological studies. Cancer. 1980 May 15;45(10):2557–2563. doi: 10.1002/1097-0142(19800515)45:10<2557::aid-cncr2820451014>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Kuratsu J., Takeya M., Yoshimura T., Ushio Y. Expression and localization of messenger RNA and protein for monocyte chemoattractant protein-1 in human malignant glioma. J Neurosurg. 1994 Jun;80(6):1056–1062. doi: 10.3171/jns.1994.80.6.1056. [DOI] [PubMed] [Google Scholar]
- Thierry F., Spyrou G., Yaniv M., Howley P. Two AP1 sites binding JunB are essential for human papillomavirus type 18 transcription in keratinocytes. J Virol. 1992 Jun;66(6):3740–3748. doi: 10.1128/jvi.66.6.3740-3748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Lichti U., Simpson S., Evans C. H., DiPaolo J. A. Leukoregulin and gamma-interferon inhibit human papillomavirus type 16 gene transcription in human papillomavirus-immortalized human cervical cells. Cancer Res. 1992 Jan 15;52(2):456–463. [PubMed] [Google Scholar]
- Woodworth C. D., Notario V., DiPaolo J. A. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. J Virol. 1990 Oct;64(10):4767–4775. doi: 10.1128/jvi.64.10.4767-4775.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Simpson S. Comparative lymphokine secretion by cultured normal human cervical keratinocytes, papillomavirus-immortalized, and carcinoma cell lines. Am J Pathol. 1993 May;142(5):1544–1555. [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976 Feb;36(2 Pt 2):794–794. [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]