Abstract
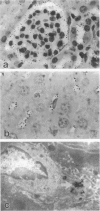
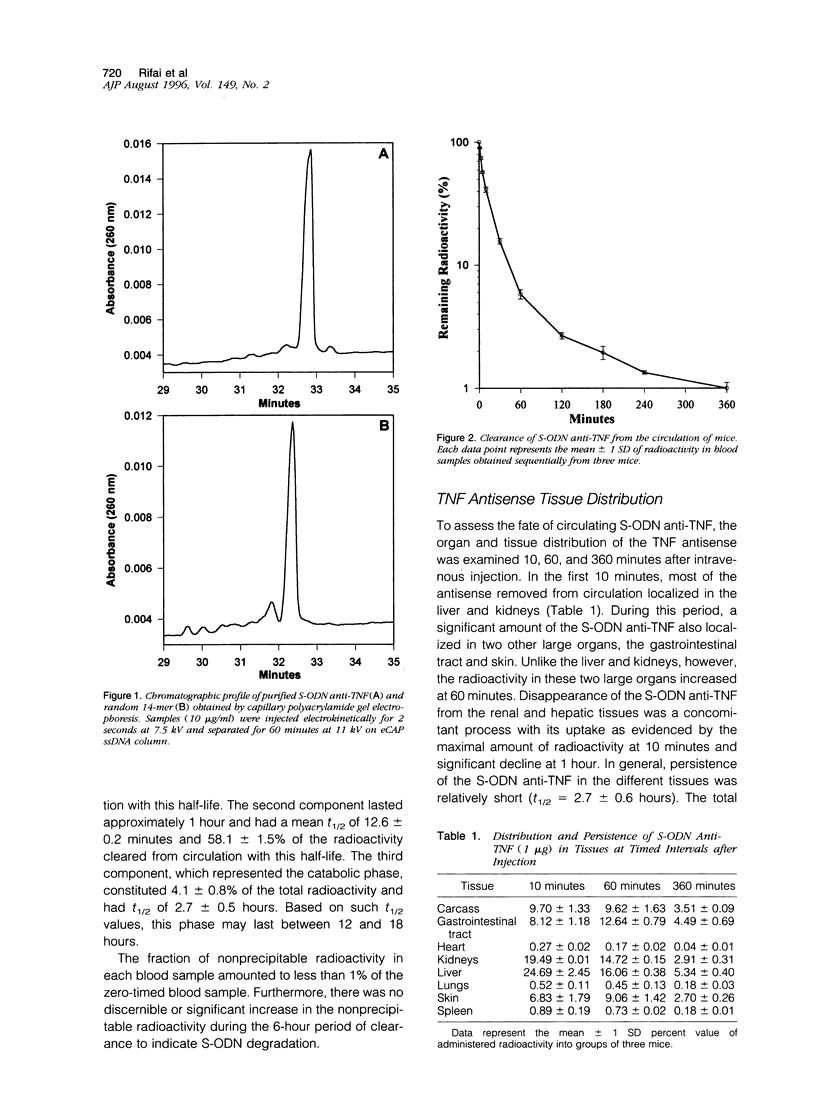
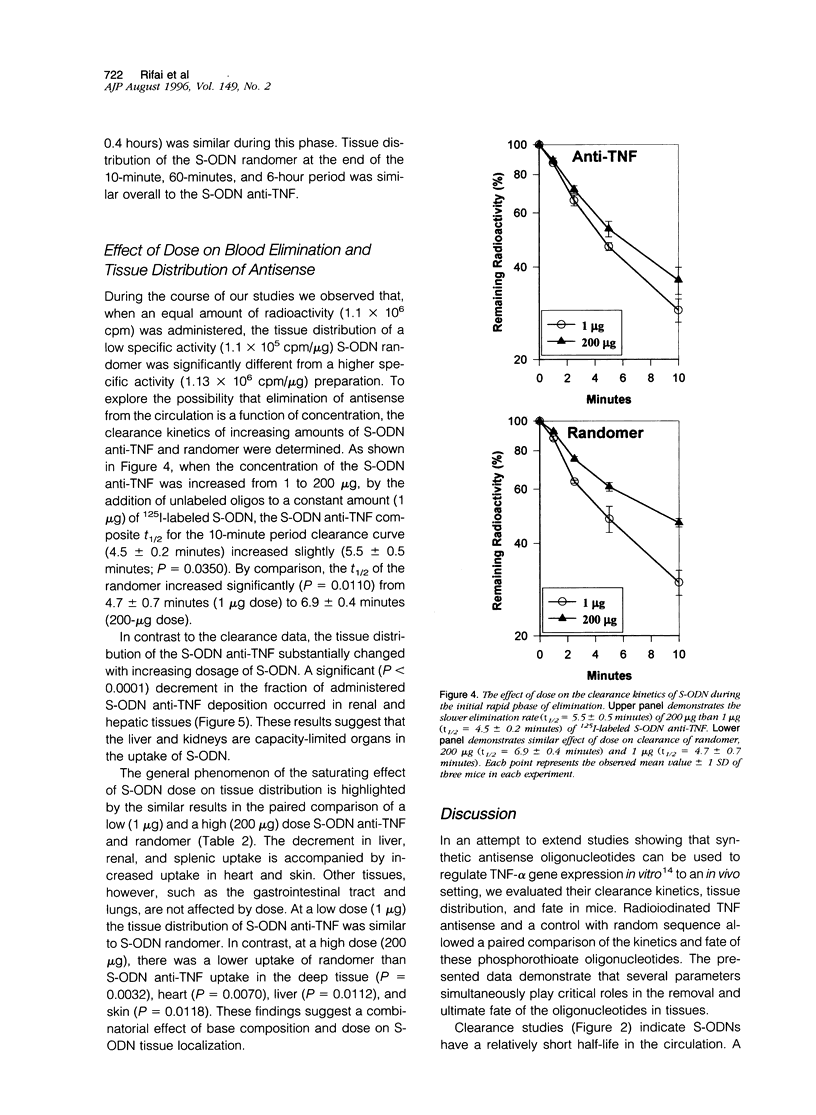
We examined the dynamics of removal from circulation, tissue distribution, and persistence of phosphorothioate oligodeoxynucleotides (S-ODN) anti-tumor-necrosis-factor and a control of random sequence (randomer) in mice. After intravenous injection, the majority (96%) of S-ODN cleared rapidly from the circulation in the first two phases. In the first phase, 37.8 +/- 2.3% of the radioactivity had a mean half-life (t1/2) of 2.0 +/- 0.4 minutes. In the second phase, 58.1 +/- 1.5% of the radioactivity cleared with t1/2 of 12.6 +/- 0.2 minutes. The catabolic phase, constituting a minor proportion (4.1 +/- 0.8% of the total radioactivity), had a mean t1/2 of 2.7 +/- 0.5 hours. At a low dose (1 microgram) tissue distribution of both S-ODN anti-tumor-necrosis-factor and randomer were similar. The liver and kidneys were the major organs involved in uptake and removal of S-ODN. Autoradiographic studies showed the liver Kupffer cells to be the major site of uptake and renal urinary space for elimination. The clearance rate from the circulation was increased with the dose of S-ODN. In contrast, the fraction of radioactivity localized in the kidneys, liver, and spleen was decreased with increase in dosage. Furthermore, at a high dose (200 micrograms), the tissue distribution of the S-ODN anti-tumor-necrosis-factor differed significantly from the randomer. These findings have general significance in showing that the liver and kidneys are the major organs for removal of S-ODN and these organs are saturable at high doses. In addition, the results have specific importance in defining different parameters, dose and base composition, that affect utilization of antisense oligonucleotides for controlling gene expression in vivo.
Full text
PDF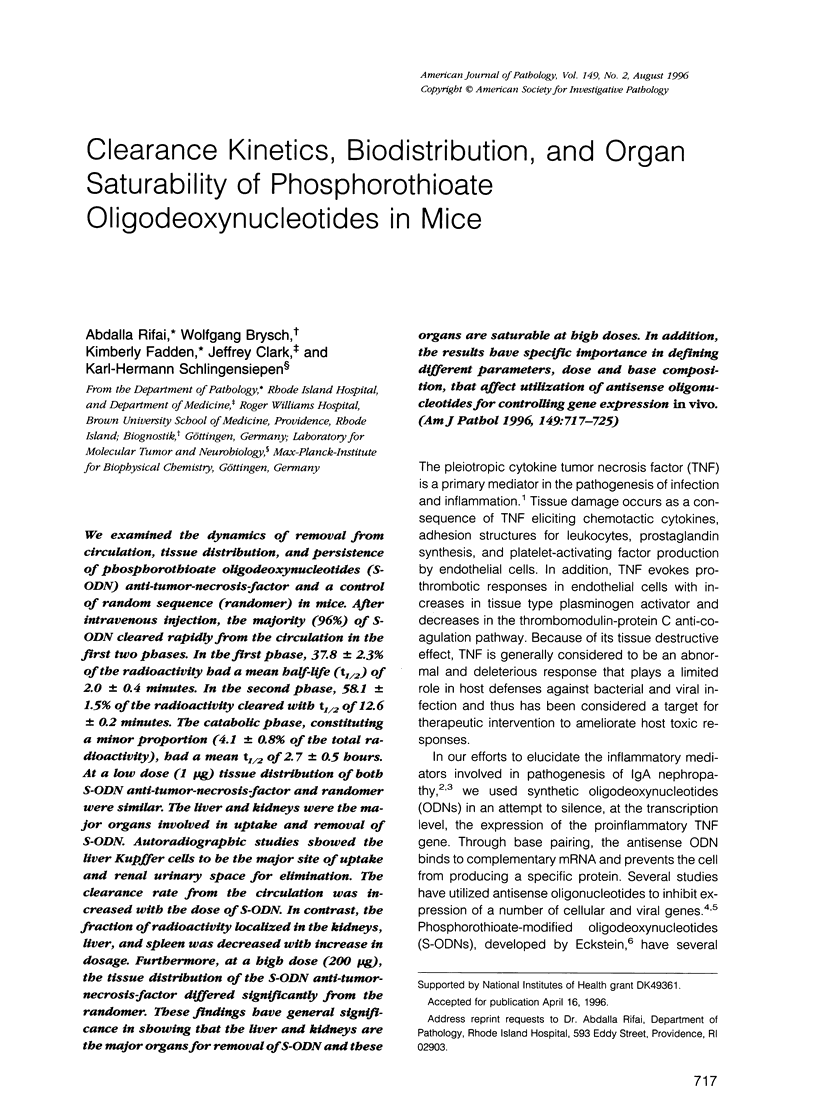



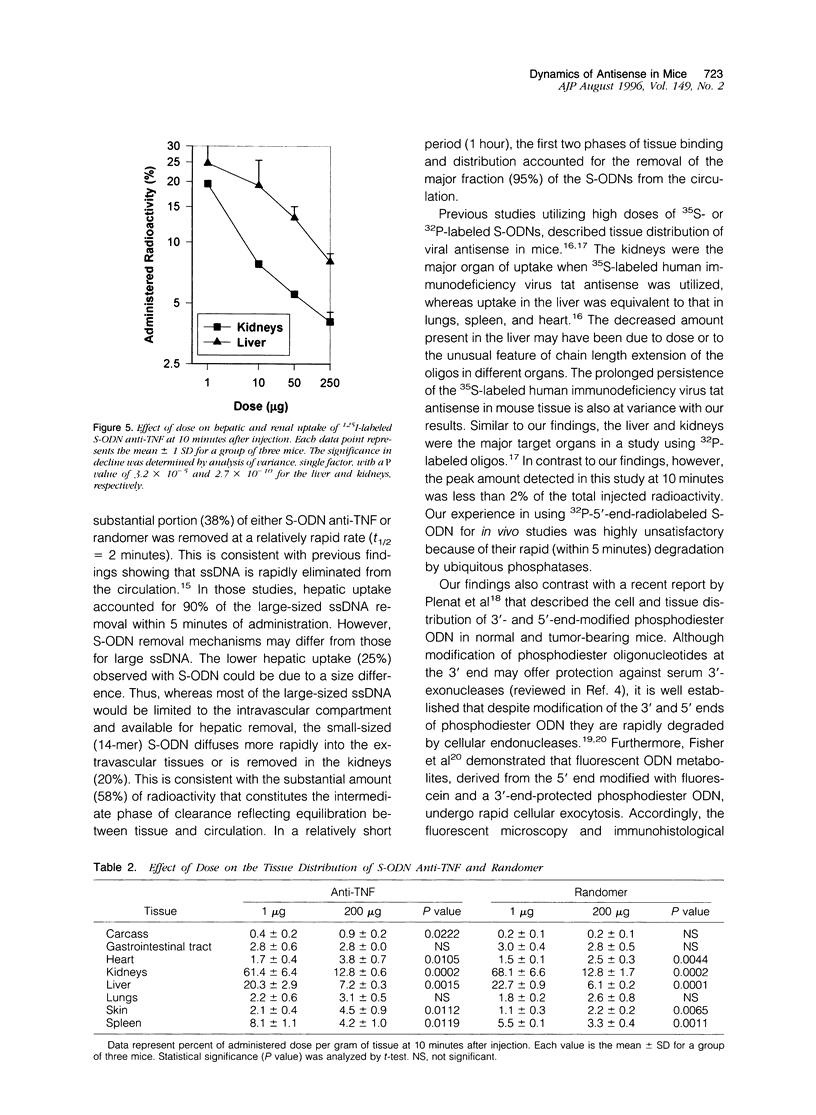


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Temsamani J., Tang J. Y. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan R., Connell Y., Fahmy B., Neckers L. Electroporation enhances c-myc antisense oligodeoxynucleotide efficacy. Nucleic Acids Res. 1993 Jul 25;21(15):3567–3573. doi: 10.1093/nar/21.15.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke R. M. In vitro toxicology and pharmacokinetics of antisense oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):609–646. [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Emancipator S. N., Lamm M. E. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989 Feb;60(2):168–183. [PubMed] [Google Scholar]
- Emlen W., Mannik M. Kinetics and mechanisms for removal of circulating single-stranded DNA in mice. J Exp Med. 1978 Mar 1;147(3):684–699. doi: 10.1084/jem.147.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Rifai A., Magilavy D., Mannik M. Hepatic binding of DNA is mediated by a receptor on nonparenchymal cells. Am J Pathol. 1988 Oct;133(1):54–60. [PMC free article] [PubMed] [Google Scholar]
- Fisher T. L., Terhorst T., Cao X., Wagner R. W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993 Aug 11;21(16):3857–3865. doi: 10.1093/nar/21.16.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi G., Watabe M., Watabe K. Organ distribution and stability of phosphorothioated oligodeoxyribonucleotides in mice. Biopharm Drug Dispos. 1992 Apr;13(3):221–227. doi: 10.1002/bdd.2510130308. [DOI] [PubMed] [Google Scholar]
- Hoke G. D., Draper K., Freier S. M., Gonzalez C., Driver V. B., Zounes M. C., Ecker D. J. Effects of phosphorothioate capping on antisense oligonucleotide stability, hybridization and antiviral efficacy versus herpes simplex virus infection. Nucleic Acids Res. 1991 Oct 25;19(20):5743–5748. doi: 10.1093/nar/19.20.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhauser G. V., Krum J. M., Rosenstein J. M. An innovative coating technique for light and electron microscopic autoradiography. J Histochem Cytochem. 1992 Jun;40(6):879–882. doi: 10.1177/40.6.1588033. [DOI] [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenat F., Klein-Monhoven N., Marie B., Vignaud J. M., Duprez A. Cell and tissue distribution of synthetic oligonucleotides in healthy and tumor-bearing nude mice. An autoradiographic, immunohistological, and direct fluorescence microscopy study. Am J Pathol. 1995 Jul;147(1):124–135. [PMC free article] [PubMed] [Google Scholar]
- Rifai A. Immunopathogenesis of experimental IgA nephropathy. Springer Semin Immunopathol. 1994;16(1):81–95. doi: 10.1007/BF00196716. [DOI] [PubMed] [Google Scholar]
- Rifai A., Mannik M. Clearance kinetics and fate of mouse IgA immune complexes prepared with monomeric or dimeric IgA. J Immunol. 1983 Apr;130(4):1826–1832. [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Witsell A. L., Schook L. B. Tumor necrosis factor alpha is an autocrine growth regulator during macrophage differentiation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4754–4758. doi: 10.1073/pnas.89.10.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]