Abstract
Employing carbohydrate ligands, which have been extensively used to block selectin function in vitro and in vivo, we have examined the involvement of such ligands in stem/progenitor cell mobilization in mice and monkeys. We found that sulfated fucans, branched and linear, are capable of increasing mature white cells in the periphery and mobilizing stem/progenitor cells of all classes (up to 32-fold) within a few hours posttreatment in a dose-dependent manner. To elicit the effect, the presence of sulfate groups was necessary, yet not sufficient, as certain sulfated hexosamines tested (chondroitin sulfates A or B) were ineffective. Significant mobilization of stem/progenitor cells and leukocytosis was elicited in selectin-deficient mice (L−/−, PE−/−, or LPE−/−) similar to that of wild-type controls, suggesting that the mode of action of sulfated fucans is not through blockade of known selectins. Other mechanisms have been entertained, in particular, the release of chemokines/cytokines, including some previously implicated in mobilization. Significant increases were documented in the levels of seven circulating chemokines/cytokines within a few hours after fucan sulfate treatment and support such a proposition. Additionally, an increase was noted in plasma metalloproteinase (MMP) 9, which might independently contribute to the mobilization process by enzymatically facilitating chemokine/cytokine release. Mobilization by sulfated polysaccharides provides a distinct paradigm in the mobilization process and uncovers an additional novel in vivo biological role for sulfated glycans. As similarly sulfated compounds were ineffective in vivo, the data also underscore the fact that polysaccharides with similar structures may elicit diverse in vivo effects.
H2emopoietic cells at various stages of differentiation are localized within the bone marrow (BM), the unique site of their development in the adult. However, terminally differentiated cells of all lineages and some stem/progenitor cells escape the BM and circulate in the peripheral blood (PB) in controlled numbers under normal, steady-state conditions. This physiologic egress of hemopoietic cells can be increased significantly by several empiric treatments, i.e., cytokines, chemokines, or chemotherapeutic agents (1). The mechanisms by which hemopoietic cells are normally released into the circulation or forced to emigrate from the BM to the PB are incompletely understood. It is generally accepted that cell adhesion molecules are principal players. Many classes of cytoadhesion molecules are present on hemopoietic stem/progenitor cells, and their cognate ligands are found within the BM microenvironment (2). Several in vitro studies have shown that many of these molecules participate in adhesion events between BM progenitor cells and stromal cells (3). Because in vitro conditions cannot faithfully mimic the complex BM microenvironment, it has been unclear whether, and to what extent, these in vitro observations are important in vivo. We have shown in animal models that function-blocking Abs to very late Ag 4 (VLA4) (4) or vascular cell adhesion molecule 1 (VCAM-1) can induce stem/progenitor mobilization (5), although many other cooperative molecules or events are required for this process (5). Furthermore, the involvement of β1 integrins in the migration of hemopoietic cells during development and the importance of α4 integrin in the establishment and maintenance of BM hemopoiesis have been shown in mice by using gene-targeting experiments (6, 7).
In addition to participating in mobilization, the VLA4/VCAM-1 pathway is an important player in the homing to the bone marrow of i.v. administered cells (ref. 8 and references therein). Although the directionality of movement is quite different in this process (transmigration through the luminal endothelium to extravascular bone marrow spaces in homing and the reverse in mobilization), the data point to the fact that the same molecules may be engaged, at least in certain steps, in both of these two processes. Furthermore, it was recently shown that, together with VLA4/VCAM-1, other molecules cooperate to ensure proper bone marrow homing. One such family of molecules is the selectins. The effect on homing was detectable only in double selectin mutants (P- and E-selectin−/−), and it revealed a novel contribution for selectins in stem/progenitor cell biology in addition to their known participation in the early steps of the extravasation cascade of mature leukocytes (9). Known ligands for the selectins include sulfated oligosaccharides, especially those rich in fucose residues, as well as glycosaminoglycans (GAGs) (10). Selectin function can be inhibited both in vitro and in vivo by the addition of these specific sulfated glycans. Fucoidan (FCN), a polysaccharide composed of sulfated fucose chains, isolated from kelp or brown seaweeds, has been shown by many studies to block selectin function both in vitro and in vivo (ref. 11 and references therein).
To investigate the involvement of selectins or carbohydrate-lectin interactions in the mobilization process, we examined the effect of FCN and similar glycans on the mobilization of mature and/or stem/progenitor cells in mice or monkeys. By using mice with genetic-ablation of selectins, we further explored whether the FCN-induced mobilization could be attributed to selectin function. Unexpectedly, we found that mobilization induced by FCN is independent of the presence of the three known selectins, P, E, and L selectins. Putative mechanisms of FCN-induced mobilization are discussed.
Materials and Methods
Reagents.
Glycans and protamine were purchased from Sigma and were cell culture grade. FCN tested negative for endotoxins (Aldevron, Fargo, ND). Granulocyte colony-stimulating factor (G-CSF) (Filgrastim Neupogen) was from Amgen Biologicals. A sulfated linear fucan was a kind gift from P. A. S. Mourão (12).
Animals.
BDF1 mice, B6,129Seletm1Hyn Selptm1Hyn mice deficient in both the P and E selectin genes (PE−/−), B6,129Selltm1Hyn mice deficient in the L selectin gene (L−/−), and their wild-type controls were purchased from The Jackson Laboratory. Mice deficient in all three selectins (LPE−/−) were produced by R. Collins and A. Beaudet, Baylor College of Medicine, Houston, TX. Mice were housed at the specific pathogen-free facility of the University of Washington approved by the American Association for the Accreditation of Laboratory Animal Care.
For mobilization studies, mice were injected i.v. with saccharides diluted in PBS with Ca2+ and Mg2+. Mice treated with G-CSF were injected twice daily with 50 μg per kg body weight for 3 days and bled at day 4. FCN was given to G-CSF-treated mice on days 2 through 4, and the mice were bled 3 h after the last treatment. Protamine-pretreated mice were injected i.v. with a total of 30 mg/kg in two injections 15 min apart, followed by FCN treatment 15 min later. PB was collected into preservative-free heparin from the retroorbital plexus of anesthetized animals.
Mobilization studies were also performed on three macaques, two Macaca nemestrina (pig-tailed monkeys) and one Macaca fascicularis. The animals were housed in the accredited Regional Primate Research Center at the University of Washington, and protocols were approved by the Institutional Review Board and by the Animal Care and Use Committee. Animals were treated with one injection of 100 mg/kg FCN or 50 or 100 mg/kg dextran sulfate in i.v. saline. PB was drawn before and after injection at times ranging from 30 min to 6 h, then a maximum of once a day for 9 days.
Clonogenic Assays.
A total WBC count from an aliquot of anticoagulated blood was measured using a Coulter counter. The remainder was cultured as previously described (5). Colonies were counted based on morphological criteria under a dissecting microscope. All colonies [burst-forming unit-erythroid (BFU-E), CFU-mixed, and granulocyte–macrophage colony-forming unit (CFU-GM)] were totaled and reported as colony-forming cells (CFC).
Colony-forming assays were performed on primate blood as described previously (4). After culturing for 12 days, colonies were counted, using a dissecting microscope, on the basis of morphological criteria. Total colonies were reported as CFC.
Assays for Colony-Forming Unit-Spleen Day 12 (CFU-S12) and for Radioprotection.
BDF1 mice treated with 100 mg/kg FCN for 3 days were bled 3 h after the last injection, and the blood was pooled. A group of untreated mice were bled for a control pool. Irradiated (12.0 Gy) recipient mice were each injected with 0.044 ml of blood per mouse from FCN-treated donors or 0.088 ml of blood per mouse from controls. Mice were killed 12 days later, and the spleens were excised and immersed in Bouin's fixative for 10 min then stored in 10% buffered formalin. The numbers of macroscopically visible colonies were counted. To assay for the presence of radioprotective cells, blood from the FCN-treated mice (0.131 ml) or untreated mice (0.264 ml) was injected into individual irradiated recipients. The animals were examined for morbidity daily for the first 14 days, then every few days.
Assays of Primate Blood.
A complete blood cell count with chemistries was performed on EDTA blood samples. Coagulation parameters [prothrombin time (PT), activated partial thromboplastin time (aPTT)] were also determined for some animals. Flow cytometry was performed on a FACSCalibur (Becton Dickinson) on cells stained with anti-CD34 Ab (anti-HPCA2, Becton, Dickinson). Chemokines and cytokine levels in plasma were analyzed by the Cytokine Analysis Laboratory, Fred Hutchinson Cancer Center, Seattle, WA. Metalloproteinases (MMP) 9 or 2 were assayed by zymogram analysis on plasma samples diluted in sample buffer and run on 10% gelatin gels using reagents from Bio-Rad. Gels were rinsed in 2.5% Triton X-100 and incubated in development buffer for 48 h. After staining (0.05% Coomassie brilliant blue G250 in 2.5:1:6.5 ethanol/acetic acid/water), cells were destained in 2-propanol/acetic acid/water (2:1:7).
Results
FCN Induces Mobilization of CFC, CFU-S, and Radioprotective Cells in Mice.
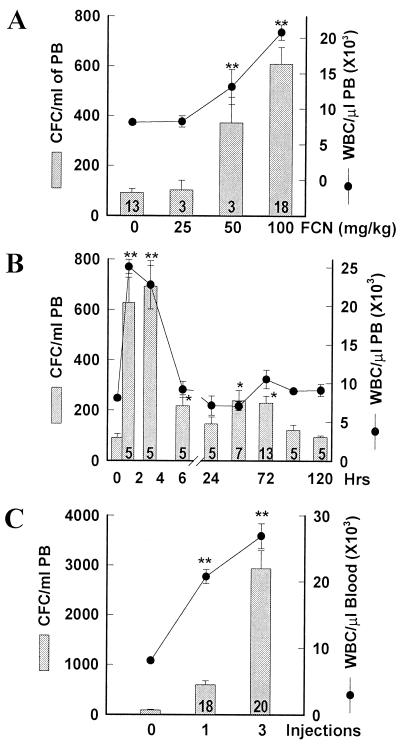
Mice were injected i.v. with FCN, and the PB was tested for the number of WBC and CFC. Single injections of different doses of FCN increased the number of CFC after 3 h in a dose-dependent manner (Fig. 1A). CFC increased an average of 6.1-fold [from 92 ± 15 (SEM) to 599 ± 73 CFC/ml, P < 0.00001] at 100 mg/kg, the highest dose tested. WBC also increased at 3 h from 8.1 ± 0.3 × 103/μl to 20.8 ± 1.1 × 103/μl (P < 0.00001). In a timed assay, the WBC at 1 h rose even more to 25.0 ± 0.9 × 103/μl PB, although the CFC did not peak until 3 h at 692 ± 84/ml (Fig. 1B). Both the WBC and CFC decreased by 6 h, and by 24 h the WBC had dropped to baseline and the CFC to 146/ml. A second, modest CFC peak at 48 to 72 h was apparent (Fig. 1B). Repeated injections, at one per day, further increased the progenitor number present in the PB at 3 h after the last injection. Three injections over 3 days increased CFC by 32-fold (Fig. 1C, P < 0.0001, n = 20) to 2929 ± 366/ml, indicating a cumulative effect, with an increase in total WBC to 26.9 ± 1.9 × 103/μl.
Figure 1.
FCN induces mobilization of CFC and WBC in mice. Mice were i.v. injected with the indicated concentration of FCN and bled after 3 h (A), or with 100 mg/kg FCN and bled at the indicated times (B), or with 100 mg/kg per day and bled 3 h after the last injection (C). Blood was assayed for CFC (bars) or WBC (circles). Number of treated mice is listed within the bar, control group, n = 13. Error bars indicate SEM; statistical analysis is by Student's t test. *, P < 0.001; ** refers to both CFC and WBC, P < 0.00001.
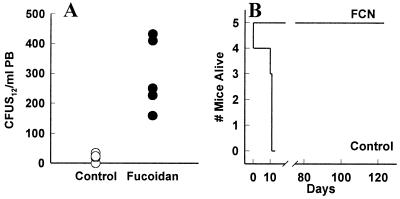
In addition to the committed progenitors, we tested FCN for its ability to mobilize more primitive progenitor cells. The average number of CFU-S12/ml present in control blood was 13 ± 2.3 (Fig. 2A). CFU-S12/ml in PB of FCN-treated mice were 295 ± 53 CFU-S12/ml, a 23-fold increase (P < 0.005). We further tested the ability of blood from FCN-treated donor mice to radioprotect and reconstitute lethally irradiated mice. All mice injected with FCN-mobilized blood (five of five) survived more than 120 days, whereas twice as much blood from untreated donors failed to save any irradiated mice past day 13 (n = 5, Fig. 2B). These assays confirm that, in addition to CFC, the cells being mobilized by FCN include radioprotective cells and likely cells required for long term repopulation (>4 mo).
Figure 2.
FCN mobilizes progenitor cells capable of radioprotection. Control mice (open circles, Control) or mice injected with 100 mg/kg per day FCN i.v. for 3 days (closed circles, FCN) were bled, and their blood was injected i.v. into irradiated mice, which were evaluated for CFU-S12 (A) or radioprotection (B) studies. Each group had five mice.
Structural Features Required for Mobilization.
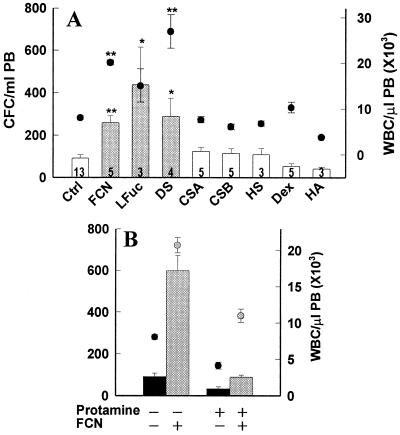
To examine the influence of structural characteristics in the ability of FCN to mobilize, we compared its effects to those of similar glycans. We first tested the necessity for the fucan to be a branched molecule. Mice were injected with a sulfated linear fucan isolated from the sea urchin Lytechinus variegatus (12). At 3 h, the CFC had increased to 10.9-fold, with a concurrent increase in WBC (Fig. 3A). These increases were similar to those seen with the branched moiety, clearly indicating that branching is not necessary for the immediate mobilization activity of sulfated fucans.
Figure 3.
Effect of structure and charge on mobilization by polysaccharides. (A) Mice were injected once (LFuc, HS) or twice (all others) with 50 mg/kg polysaccharides 24 h apart, then bled 3 h after the last injection and evaluated for CFC (bars) or WBC (circles). Control (Ctrl), fucoidan (FCN), linear fucan (LFuc), dextran sulfate (DS), chondroitin sulfate A or B (CSA, CSB), heparan sulfate (HS), dextran (Dex), hyaluronic acid (HA). Number of treated mice is listed within each bar. (B) Control mice (Left, untreated n = 13, FCN alone n = 18) or mice (Right, n = 5 per group) treated with protamine alone (black bars and circles) or cotreated with 100 mg/kg FCN (gray bars and circles). Error bars and statistics as in Fig. 1. *, P < 0.001; **, P < 0.00001.
We next tested a sulfated polymer of hexose units, dextran sulfate (DS). Two injections (50 mg/kg each) of DS in mice mobilized CFC and WBC each more than 3-fold, similar to 50 mg/kg FCN (Fig. 3A). Furthermore, when 50 mg/kg FCN was combined with 50 mg/kg DS, an additive increase in CFC of 10-fold was seen. These results indicate that the ability to mobilize is not limited to fucose residues.
Additionally, we examined GAGs with sulfation similar in amounts and linkages to that of the fucan for their ability to mobilize. Chondroitin sulfate A (CS-A) or chondroitin sulfate B (CS-B) at comparable doses failed to mobilize either WBC or CFC (Fig. 3A), as did a single injection of heparan sulfate (HS) from porcine intestinal mucosa in preliminary studies. These results raise the possibility that sulfated forms of the simple hexoses act similarly to induce mobilization, whereas hexosamines do not.
The 2,6-sulfation of FCN, DS, and other glycans is considered to be important for their binding and function. Therefore, to test the need for sulfation in mobilization, mice were treated with a dextran of the same molecular weight as the mobilizing DS. In contrast to the DS, the dextran induced no mobilization of either WBC or CFC. Similarly, hyaluronic acid, a nonsulfated GAG (Fig. 3A), failed to mobilize.
Whether a negative charge imparted to the saccharide molecule by the sulfate group was needed for mobilization was tested next. To neutralize FCN, mice were first treated with protamine, known also to neutralize heparin, and within 15 min administered a single dose of FCN. With FCN alone, the CFC increased 6-fold at 3 h (Figs. 1A and 3B). This increase was virtually abolished in the presence of protamine (Fig. 3B). The increase in WBC was also significantly inhibited (P < 0.001). Protamine alone decreased both the CFC and WBC below baseline levels, yet the fold increase in CFC in animals treated with both FCN and protamine was 2.7 as compared with 6.9 for FCN alone.
FCN Induces Mobilization in Selectin-Deficient Mice.
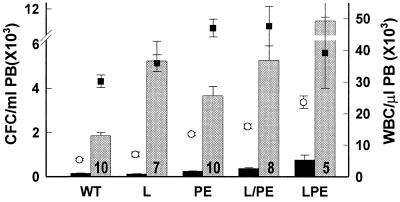
FCN has been reported to inhibit selectin function in vitro and in vivo. To definitively establish whether any or all of the selectins are involved in FCN-induced mobilization, we tested the glycan in mice deficient in one, two, or all three selectin genes. After three i.v. injections, wild-type control mice had a 13-fold increase in CFC from 145 ± 18/ml to 1862 ± 124/ml PB, with a concurrent 6-fold increase of WBC from 5.4 × 103 to 30.2 × 103/μl. After a similar regimen of FCN treatment in mice lacking the active gene for L-selectin (the selectin most sensitive to FCN in vitro), CFC increased from 152 ± 29 to 5997 ± 554 CFC/ml, with a concurrent increase in WBC from 5.5 ± 0.7 to 35.9 ± 2.6 × 103/μl PB (Fig. 4). As compared with wild-type controls, mice deficient for P- and E-selectin genes exhibited elevated baseline levels of WBC (13.5 ± 0.5 × 103/μl), similar to reported values (9, 13), as well as elevated CFC (236 ± 23/ml), not previously reported. These values increased further after treatment with FCN to ratios similar to those seen in the control mice, CFC increased 15-fold to 3669 ± 424/ml and WBC increased 4-fold to 47 ± 2.8 × 103/μl PB (Fig. 4).
Figure 4.
FCN induces mobilization in the absence of selectins. Control mice (WT) and L-selectin−/− (L), P- and E-selectin−/− (PE) themselves or transplanted with L−/− BM cells (L/PE), or L-, P-, and E-selectin−/− (LPE) mice were prebled (black bars and open circles) and a week later injected i.v. three times with 100 mg/kg FCN 24 h apart and bled 3 h after the last injection (gray bars and closed squares). Number of mice is listed within each treated bar. Error bars and statistics as in Fig. 1. All CFC values in treated groups were statistically significantly different from the values in nontreated groups (P < 0.01 to P < 0.0001).
To rule out that some compensation was present in the double or single selectin knock-out mice, we assessed mobilization in two other groups of mice: first, PE−/− mice, which were transplanted with bone marrow cells from L−/− mice, leaving only the P-selectin present on blood cells, and, second, LPE−/− mice deficient for all three selectins. After FCN treatment, the transplanted mice had similar increases in CFC from 416 ± 63 to 6,381 ± 1,476 CFC/ml (15-fold) and WBC from 17 ± 1.2 × 103/μl to 52.8 ± 7.5 × 103 WBC/μl (3.1-fold, Fig. 4). As expected (13), LPE−/− had even higher baseline levels than PE−/− (23.6 ± 2 × 103/μl WBC and 759 ± 217 CFC/ml), yet the ratio of increase in the CFC in these mice after treatment remained at 15-fold (11,116 ± 3,336 CFC/ml, Fig. 4). Taken together, these data clearly establish that engagement of P-, E-, or L-selectin is not necessary for FCN to induce mobilization of stem/progenitor cells into the blood stream, thereby suggesting a selectin-independent mode of action.
FCN Induces Mobilization of CFC in Nonhuman Primates.
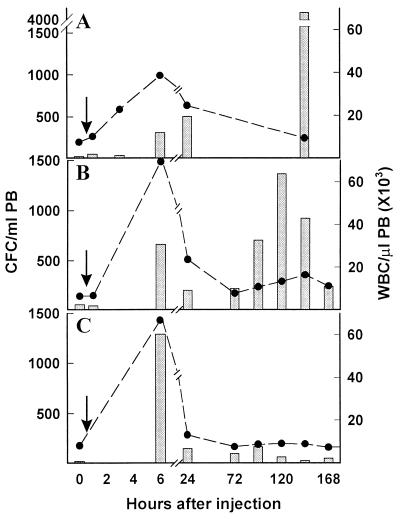
FCN was also tested for its ability to mobilize CFC in two species of macaque. Two M. nemestrina and one M. fascicularis were each treated i.v. with 100 mg/kg FCN in saline and examined over time. After one injection, the CFC and WBC of one M. nemestrina increased 11- and 5.2-fold, respectively, at 6 h, maintaining the increase through day 1 (Fig. 5A). PB mononuclear cells, stained with anti-CD34 and measured by flow cytometry, also increased at 6 h from 0.2% to 6.4% (data not shown). The second M. nemestrina increased CFC 18-fold to 714/ml and WBC 3-fold to 23.8 × 103/μl at 6 h. Likewise, FCN injection in the M. fascicularis increased CFC 11-fold and WBC 8-fold at 6 h, and still maintained some increase at 24 h (Fig. 5B). In addition to the 6-h peak, a secondary peak was seen in the M. nemestrina 5 days after FCN treatment (Fig. 5A). This peak appeared to be more gradual, lasted longer than the primary peak at 6 h (Fig. 5B), and was, in fact, higher than the original peak in both monkeys, increasing up to 140- and 23-fold, respectively.
Figure 5.
Mobilization of CFC and WBC in monkeys. Animals were bled at time 0, then as indicated after i.v. injection (arrow) and tested for CFC (bars) or WBC (circles). (A and B) M. nemestrina (A) or M. fascicularis (B) each treated with 100 mg/kg FCN. (C) M. fascicularis treated with 50 mg/kg DS.
We also treated the M. fascicularis (shown in Fig. 5B) 8 wk later with 50 mg/kg DS, and observed increases in CFC (26-fold) and WBC (8-fold) at 6 h (Fig. 5C) similar to those observed in FCN treatment, but no second peak was observed (Fig. 5C). Because both compounds have been reported to have anticoagulant activity, coagulation studies were performed. At 6 h posttreatment with 100 mg/kg FCN or 50 mg/kg DS, the PT and aPTT values were elevated (data not shown), although no evidence of major bleeding was observed, and the values returned to baseline by 24 h. The M. nemestrina (shown in Fig. 5A) was also treated later with 100 mg/kg DS and showed a 20-fold increase of CFC at 6 h with some increase at day 5 (data not shown), but the animal exhibited signs of bleeding; it was transfused, and the study was discontinued.
FCN Induces Increased Levels in Plasma of Cytokines, Chemokines, and MMPs in Nonhuman Primates.
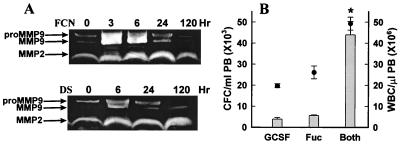
We next explored whether we could document any increases in circulating molecules previously implicated in mobilization, i.e., chemokines, cytokines, or MMPs. Very high increases at 3 to 6 h posttreatment were apparent in levels of monocyte chemotactic protein 1 (MCP-1), IL-6, IL-8, macrophage CSF (M-CSF), and G-CSF in monkeys treated with FCN (Table 1). Although a tumor necrosis factor-α (TNF-α) increase was not documented, levels of soluble TNF receptor 1 (sTNFR 1), an indicator of TNF activity, did increase. Soluble kit ligand (KL) also increased and was the only cytokine maintained at high levels beyond 24 h. Most of these cytokines have been previously shown (14–20) to play a role in the trafficking of progenitors or mature cells. Other cytokines tested [IL-1α, IL-1β, GM-CSF, and macrophage inflammatory protein-1α (MIP-1α)] showed no increases (data not shown). Zymograms of the plasma of FCN-treated monkeys showed an increase in levels of the metalloproteinase MMP9 at 3 and 6 h, returning to baseline levels at 24 h (Fig. 6A), similar to the levels of cytokines and chemokines.
Table 1.
Effect of sulfated glycans on plasma levels of cytokines and chemokines
| Time,* h | Level, pg/ml of plasma
|
||||||
|---|---|---|---|---|---|---|---|
| IL-6 | IL-8 | MCP-1 | M-CSF | G-CSF | sTNFR1 | KL | |
| 100 mg/kg FCN in M. nemestrina 1 (Fig. 5A) | |||||||
| 0 | 2 | 38 | 257 | 189 | 0 | 186 | 1,169 |
| 1 | 707 | 857 | 492 | 1,021 | 0 | 1,508 | ND |
| 3 | 4,164 | 2,727 | 86,469 | 3,363 | 1,767 | 1,464 | 3,944 |
| 6 | 572 | 1,868 | 12,059 | 815 | 639 | 612 | 4,835 |
| 24 | 16 | 29 | 818 | 638 | 15 | 634 | 5,245 |
| 144 | 0 | 13 | 165 | 24 | 0 | 228 | 1,388 |
| 100 mg/kg FCN in M. nemestrina 2 | |||||||
| 0 | 0 | 52 | 227 | 0 | 0 | 256 | 673 |
| 6 | 2,741 | 4,136 | 75,127 | 427 | 2,097 | 916 | 3,856 |
| 100 mg/kg FCN in M. fascicularis (Fig. 5B) | |||||||
| 0 | 0 | 41 | 171 | 0 | 0 | 192 | 822 |
| 0.5 | 4 | 618 | 909 | 161 | 0 | 2,191 | 1,652 |
| 6 | 1,677 | 554 | 9,194 | 344 | 422 | 1,118 | ND |
| 24 | 7 | 21 | 299 | 63 | 0 | 226 | 1,005 |
| 72 | 0 | 84 | 240 | 0 | 0 | 164 | 456 |
| 96 | 0 | 81 | 179 | 15 | 0 | 174 | 2,864 |
| 120 | 9 | 82 | 168 | 33 | 0 | 210 | 708 |
| 144 | 0 | 106 | 228 | 0 | 0 | 188 | 1,228 |
| 50 mg/kg DS in M. fascicularis (Fig. 5C) | |||||||
| 0 | 0 | 72 | 197 | 0 | 0 | 179 | ND |
| 6 | 7 | 125 | 1,226 | 91 | 0 | 330 | ND |
| 100 mg/kg DS in M. nemestrina | |||||||
| 0 | 0 | 64 | 213 | 41 | 0 | 178 | 1,169 |
| 6 | 1 | 306 | 603 | 207 | 0 | 272 | 673 |
| 24 | 1 | 21 | 270 | 163 | 0 | 355 | 845 |
| 120 | 0 | 77 | 182 | 83 | 0 | 309 | 822 |
A 0 indicates values less than lower limit detected. ND indicates this sample was not tested. Values 2-fold or higher over baseline are in bold.
Animals were bled at 0 time and at indicated times after a single i.v. injection.
Figure 6.
(A) Zymograms of monkey plasmas. Samples were drawn from monkeys at the indicated times after treatment with 100 mg/kg FCN or DS and run on a gel containing 10% gelatin. (B) Mobilization of CFC (bars) and WBC (circles) in mice after treatment with G-CSF, FCN, or both. G-CSF or Fuc, n = 5, both, n = 4 mice. Error bars and statistics as in Fig. 1. * Refers to both CFC and WBC, P < 0.001 as compared with G-CSF or FCN.
In the DS-treated monkeys, plasma levels of MCP-1, IL-6, IL-8, M-CSF, and sTNFR1 increased, but far less than in FCN-treated monkeys (Table 1), and kit ligand did not increase. The MMP9 levels also increased in the DS-treated monkey, although not as high as with FCN (Fig. 6A).
Because G-CSF levels were increased significantly with FCN treatment in the monkey, we investigated the possibility that G-CSF in synergy with the chemokines was primarily responsible for FCN-induced mobilization. When used alone in mice, G-CSF over 3 days mobilized CFC 41-fold, and FCN alone 61-fold (Fig. 6B). However, administration of both G-CSF and FCN increased the CFC an astounding 483-fold over baseline, 11 times that of the G-CSF alone, and 8 times that of the FCN alone. These results demonstrate an ability of FCN to work synergistically with G-CSF, maximizing the mobilization yield, and indirectly suggest that FCN-induced mobilization may not be primarily due to increases in G-CSF.
Discussion
Although FCN is not a physiologic ligand, it has been used extensively to block selectin function because of its structural similarity to natural ligands recognized by selectins. In vitro FCN is known to bind to L-selectin, and its binding to intact leukocytes is inhibited by L-selectin Abs and vice versa (21, 22). In vivo, FCN blocks leukocyte rolling in a dose-dependent manner, interfering with various inflammatory responses in several animal models (23–26). Inhibition of leukocyte rolling and a subsequent increase in peripheral WBC induced by FCN was similar to that observed in anti-CD18-treated rabbits (25). The increase in both polymorphonuclear leukocytes (PMNs) and mononuclear cells appeared gradually, and was attributed to the prevention of the normal elimination of PMNs from circulation and of the homing process of mononuclear cells to peripheral lymphoid tissues. This conclusion is supported by previous findings of inhibition of lymphocyte homing in mice (27) and assumes that normal elimination of PMNs involves adhesion pathways similar to those used when leukocytes are recruited to inflammatory tissues.
Our in vivo FCN treatment, like previous studies (25), resulted in leukocytosis at 1 to 3 h posttreatment in mice, and at 3 to 6 h in monkeys after a single injection. Furthermore, in parallel with the leukocyte increase, a significant mobilization of stem/progenitor cells was seen, a novel finding with FCN treatment. This increase involved all classes of progenitor cells, i.e., lineage-committed progenitors (CFU-GM, BFU-E, CFU-Meg), CFU-S12 (Fig. 2), and likely long-term repopulating cells, although the latter were not rigorously addressed. As reported in previous studies, we hypothesized that FCN exerted its effects by blocking selectin function. However, our extensive data with various selectin-deficient models show that such a possibility is not likely. Namely, the single L-selectin-deficient mice, the double P- and E-selectin-deficient mice, as well as the triple selectin-deficient mice all responded as well as their wild-type controls. Therefore, both the leukocytosis and the mobilization of stem/progenitor cells in these mice appear to be by a mechanism that does not involve at least the known selectins.
In view of the findings in selectin-deficient mice, to explain the leukocytosis and the stem cell mobilization process several possibilities were entertained. First, it is possible that FCN binds to leukocytes in a selectin-independent way. Since high concentrations of FCN are needed to exert its effects, interactions with low-affinity binding sites on either leukocytes or stem/progenitor cells are possible. Such interactions may involve unidentified lectins on these cells, or even on endothelial or stromal cells within the bone marrow microenvironment. FCN is capable of binding to nonhemopoietic cells lacking selectins (at both high and low affinity binding sites) and modulating production of cellular proteins (28). A similar interaction may be involved in mobilization.
Alternatively, and more likely, FCN could compete with proteoglycans found on the extracellular matrix (ECM) or on stromal/endothelial cells within the bone marrow environment. Chemokines/cytokines bound by proteoglycans and their constituent GAGs are present at high concentrations within the BM, where they exert important functional influences on the development and differentiation of hemopoietic cells, as well as their migration in and out of the BM (28). Proteoglycans within the BM environment carry mainly HS and CS, highly sulfated glycans, with which FCN may compete in the binding of chemokines/cytokines, and in fact, FCN has been shown to compete with heparin at a higher affinity (29). Such a putative mechanism appears attractive and finds support in our documentation of dramatic increases in several circulating chemokines/cytokines within the first few hours of treatment. Of special importance is the increase in particular chemokines/cytokines that have been previously implicated in mobilization. These include IL-8 (17), and IL-6 (20), also G-CSF, M-CSF, and KL (14, 19). Additionally, there was an increase in MMP9, which was also previously reported to be an important mediator of IL-8- or G-CSF-induced mobilization (30). Its enzymatic activity may additionally have a role in releasing matrix-bound growth factors as a consequence of extracellular matrix degradation. It is unlikely that the increases that we have seen in chemokines are due to endotoxin contamination, as our preparation was found to be endotoxin-free, and no initial neutropenia was seen after treatment, as is observed postendotoxin (31). In general, however, a quantitative relationship between mobilization and cytokine increases is not seen, and efforts should be made to identify additional molecules.
It is of extreme interest that other sulfated polysaccharides, especially CS-B or HS from a single source, which are known to have anticoagulant properties (32), did not induce significant leukocytosis or mobilization. This observation would suggest that a backbone of distinct sugar residues with specific sulfation patterns is required to elicit mobilization effects, differing from those required for anticoagulation (32). If the etiologic mechanism turned out to be the release of chemokines/cytokines, our data further support the emerging principle that the carbohydrate sequences and/or sulfation patterns of GAGs within the BM that sequester these chemokines/cytokines are specific for these factors (33–36). Presumably as a result of the specificity in binding patterns, several in vitro and in vivo data suggest that structurally similar compounds may elicit distinct functional effects (37). This concept is taken a step further with in vivo mobilization induced by FCN and DS, in contrast to CS-A and CS-B. Moreover, although both induced mobilization, their responses differed in terms of anticoagulation potency, the restricted repertoire of cytokine release (Table 1), and the ability to induce in monkeys a second wave of mobilization.
Finally, it is noteworthy that certain previous studies have reported mobilization induced by carbohydrates [i.e., DS (38), particulate β-glucan (39), and PGG-glucan (40)]; however, their effects have not been dissociated from selectin-dependent interactions, and chemokine/cytokine increases were not noted. Although the mechanism of action of sulfated glycans used in the present study remains to be determined, our data further underscore a distinctive and structurally restricted in vivo specificity in their biological action.
Acknowledgments
We are grateful to Dr. P. A. S. Mourão for supplying the linear fucan. The expert technical assistance of Sarina Elliott and Vivian Zafiropoulos is gratefully acknowledged. This work was supported by National Institutes of Health Grants AI32177, HL46557, HL58734, and RR00166.
Abbreviations
- FCN
fucoidan
- DS
dextran sulfate
- CS-A or CS-B chondroitin sulfate A or B
CFC, colony-forming cells
- CFU-S
colony forming unit-spleen cells
- MMP
metalloproteinase
- PB
peripheral blood
- G-CSF
granulocyte colony-stimulating factor
- GAG
glycosaminoglycan
- HS
heparan sulfate
- VLA
very late Ag
- VCAM
vascular cell adhesion molecule
- TNF
tumor necrosis factor
- BM
bone marrow
References
- 1.Papayannopoulou T. Ann N Y Acad Sci. 1999;872:187–197. doi: 10.1111/j.1749-6632.1999.tb08464.x. [DOI] [PubMed] [Google Scholar]
- 2.Long M W. Exp Hemat. 1992;20:288–301. [PubMed] [Google Scholar]
- 3.Yoder M C, Williams D A. Exp Hemat. 1995;23:961–967. [PubMed] [Google Scholar]
- 4.Papayannopoulou T, Nakamoto B. Proc Natl Acad Sci USA. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papayannopoulou T, Priestley G V, Nakamoto B. Blood. 1998;91:2231–2239. [PubMed] [Google Scholar]
- 6.Hirsch E, Iglesias A, Potocnik A J, Hartmann U, Fassler R. Nature (London) 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo A G, Yang J T, Rayburn H, Hynes R O. Immunity. 1999;11:555–566. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 8.Papayannopoulou T, Craddock C. Acta Haematol. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- 9.Frenette P S, Subbarao S, Mazo I B, von Andrian U H, Wagner D D. Proc Natl Acad Sci, USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varki A. J Clin Invest. 1997;100:S31–S35. [PubMed] [Google Scholar]
- 11.Arfors K E, Ley K. J Lab Clin Med. 1993;121:201–202. [PubMed] [Google Scholar]
- 12.Mulloy B, Ribeiro A, Alves A, Vieira R P, Mourão P A S. J Biol Chem. 1994;269:22113–22123. [PubMed] [Google Scholar]
- 13.Robinson S D, Frenette P S, Rayburn H, Cummiskey M, Ullman-Culleré M, Wagner D D, Hynes R O. Proc Natl Acad Sci USA. 1999;96:11452–11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.To L B, Haylock D N, Simmons P J, Juttner C A. Blood. 1997;89:2233–2258. [PubMed] [Google Scholar]
- 15.Vose J M, Armitage J O. J Clin Oncol. 1995;4:1023–1035. doi: 10.1200/JCO.1995.13.4.1023. [DOI] [PubMed] [Google Scholar]
- 16.Theilgaard-Monch K, Raaschou-Jensen K, Andersen H, Russell C A, Vindeløv L, Jacobsen N, Dickmeiss E. Bone Marrow Transplant. 1999;23:243–249. doi: 10.1038/sj.bmt.1701579. [DOI] [PubMed] [Google Scholar]
- 17.Opdenakker G, Fibbe W E, van Damme J. Immunol Today. 1998;19:182–189. doi: 10.1016/s0167-5699(97)01243-7. [DOI] [PubMed] [Google Scholar]
- 18.Laterveer L, Lindley I J D, Heemskerk D P M, Camps J A J, Pauwels E K J, Willemze R, Fibbe W E. Blood. 1996;87:781–788. [PubMed] [Google Scholar]
- 19.Andrews R G, Bridell R A, Knitter G H, Opie T, Bronsden M, Myerson D, Applebaum F R, McNiece I K. Blood. 1994;84:800–810. [PubMed] [Google Scholar]
- 20.Pettengel R, Luft T, de Wynter E, Coutinho L, Young R, Fitzsimmons L, Scarffe J H, Testa N G. Br J Haematol. 1995;89:237–242. doi: 10.1111/j.1365-2141.1995.tb03295.x. [DOI] [PubMed] [Google Scholar]
- 21.Yednock T A, Stoolman L M, Rosen S D. J Cell Biol. 1987;104:713–723. doi: 10.1083/jcb.104.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yednock T A, Butcher E C, Stoolman L M, Rosen S D. J Cell Biol. 1987;104:725–731. doi: 10.1083/jcb.104.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindbom L, Xie X, Raud J, Hedqvist P. Acta Physiol Scand. 1992;146:415–421. doi: 10.1111/j.1748-1716.1992.tb09442.x. [DOI] [PubMed] [Google Scholar]
- 24.Ley K, Linnemann G, Meinen M, Stoolman L M, Gaehtgens P. Blood. 1993;81:177–185. [PubMed] [Google Scholar]
- 25.Granert C, Raud J, Xie X, Lindquist L, Lindbom L. J Clin Invest. 1994;93:929–936. doi: 10.1172/JCI117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimaoka M, Ikeda M, Iida T, Taenaka N, Yoshiya I, Honda T. Am J Respir Crit Care Med. 1996;153:307–311. doi: 10.1164/ajrccm.153.1.8542135. [DOI] [PubMed] [Google Scholar]
- 27.Spangrude G J, Braaten B A, Daynes R A. J Immunol. 1984;132:354–362. [PubMed] [Google Scholar]
- 28.Verfaille C M. In: Hematology, Basic Principles and Practice. Hoffman R, Benz E J, Shattil S J, Furie B, Cohen H J, Silberstein L E, McGlave P, editors. New York: Churchill Livingstone; 2000. pp. 143–154. [Google Scholar]
- 29.Vischer P, Buddecke E. Eur J Cell Biol. 1991;56:404–414. [PubMed] [Google Scholar]
- 30.Janowska-Weiczorek A, Marquez L A, Nabholtz J M, Cabuhat M L, Montaño J, Chang H, Rozmus J, Russell J A, Edwards D R, Turner A R. Blood. 1999;93:3379–3390. [PubMed] [Google Scholar]
- 31.Vos O, Buurman W A, Ploemacher R E. Cell Tissue Kinet. 1972;5:467–479. doi: 10.1111/j.1365-2184.1972.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereira M S, Mulloy B, Mourão P A S. J Biol Chem. 1999;274:7656–7667. doi: 10.1074/jbc.274.12.7656. [DOI] [PubMed] [Google Scholar]
- 33.Kuschert G S V, Coulin F, Power C A, Proudfoot A E I, Hubbard R E, Hoogewerf A J, Wells T N C. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 34.Spillman D, Witt D, Lindahl U. J Biol Chem. 1998;273:15487–15493. doi: 10.1074/jbc.273.25.15487. [DOI] [PubMed] [Google Scholar]
- 35.Stringer S E, Gallagher J T. J Biol Chem. 1997;272:20508–20514. doi: 10.1074/jbc.272.33.20508. [DOI] [PubMed] [Google Scholar]
- 36.Witt D P, Lander A D. Curr Biol. 1994;4:394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl U, Kusche-Gullberg M, Kjellen L. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 38.van der Ham A C, Benner R, Vos O. Cell Tissue Kinet. 1977;10:387–397. doi: 10.1111/j.1365-2184.1977.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 39.Patchen M L, MacVittie T J. Acta Haematol. 1983;70:281–288. doi: 10.1159/000206753. [DOI] [PubMed] [Google Scholar]
- 40.Patchen M L, Liang J, Vaudrain T, Martin T, Melican D, Zhong S, Stewart M, Quesenberry P J. Stem Cells. 1998;16:208–217. doi: 10.1002/stem.160208. [DOI] [PubMed] [Google Scholar]