Abstract
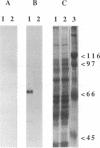
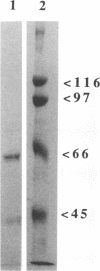
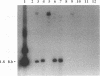
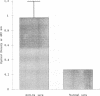
The group A streptococcal sequela acute rheumatic fever (ARF) has been associated with immunological cross-reactivity between streptococcal and heart proteins. To identify Streptococcus pyogenes genes that encode a myosin cross-reactive antigen(s) recognized by ARF sera, a genomic library from an emm deletion strain (T28/51/4) was screened with a single ARF serum. A positively identified lambda EMBL3 clone (T.2.18) produced a protein which reacted with myosin-specific antibodies affinity purified from individual ARF sera. The recombinant protein was initially estimated to be 60 kDa in size by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; however, upon sequence analysis it had a molecular mass equivalent to 67 kDa. Sera from patients with streptococcal infections, acute glomerulonephritis, and ARF were reactive with the recombinant 67-kDa protein. However, individual sera from healthy persons were negative or demonstrated low levels of reactivity with the 67-kDa antigen. The gene encoding the 67-kDa myosin-cross-reactive antigen was subcloned, and its nucleotide sequence was determined by using a combined strategy of DNA sequencing of the cloned gene and N-terminal amino acid sequencing of the protein expressed in Escherichia coli. The amino-terminal sequence deduced from the nucleotide sequence of an open reading frame was identical to that determined from the 67-kDa protein expressed in E. coli. The gene encoded 590 amino acids with a calculated molecular weight of 67,381. No cleavable signal peptide was detected with the 67-kDa protein expressed in E. coli. The deduced amino acid sequence of the 67-kDa protein did not exhibit significant similarity to any known streptococcal proteins. However, it was found to be 19% identical and 62% similar over 151 amino acid residues to the beta chain of mouse major histocompatibility complex class II antigen (I-Au). Similar degrees of homology to the beta chains of other murine and human class II haplotypes were found. Mouse anti-IA sera reacted with the recombinant 67-kDa protein about five times more strongly than normal mouse sera in the enzyme-linked immunosorbent assay. Southern hybridization experiments using a probe for the gene encoding the 67-kDa protein showed that the gene was present and conserved among pathogenic groups A, C, and G of streptococci. These data suggest that the streptococcal protein, which is distinct from the M protein, may have structural features in common with the beta chain of the class II antigens, as well as myosin, and may play an important role in the pathogenesis of streptococcal infections.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita M., Sasaki S., Matsuyama S., Mizushima S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J Biol Chem. 1990 May 15;265(14):8164–8169. [PubMed] [Google Scholar]
- Amoils B., Morrison R. C., Wadee A. A., Marcus R., Ninin D., King P., Sareli P., Levin S., Rabson A. R. Aberrant expression of HLA-DR antigen on valvular fibroblasts from patients with active rheumatic carditis. Clin Exp Immunol. 1986 Oct;66(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- Barnett L. A., Cunningham M. W. A new heart-cross-reactive antigen in Streptococcus pyogenes is not M protein. J Infect Dis. 1990 Oct;162(4):875–882. doi: 10.1093/infdis/162.4.875. [DOI] [PubMed] [Google Scholar]
- Barnett L. A., Cunningham M. W. Evidence for actinlike proteins in an M protein-negative strain of Streptococcus pyogenes. Infect Immun. 1992 Sep;60(9):3932–3936. doi: 10.1128/iai.60.9.3932-3936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Cleary P. P. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J Biol Chem. 1990 Feb 25;265(6):3161–3167. [PubMed] [Google Scholar]
- Collins C. M., Kimura A., Bisno A. L. Group G streptococcal M protein exhibits structural features analogous to those of class I M protein of group A streptococci. Infect Immun. 1992 Sep;60(9):3689–3696. doi: 10.1128/iai.60.9.3689-3696.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Cunningham M. W., Krisher K., Graves D. C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect Immun. 1984 Oct;46(1):34–41. doi: 10.1128/iai.46.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Krisher K., Graves D. C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect Immun. 1984 Oct;46(1):34–41. doi: 10.1128/iai.46.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Fenderson P. G., Ho M. K., Beachey E. H., Dale J. B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. 1989 Oct 15;143(8):2677–2683. [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Talaber L. R., Harley J. B., Ayoub E. M., Muneer R. S., Chun L. T., Reddy D. V. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J Immunol. 1988 Oct 15;141(8):2760–2766. [PubMed] [Google Scholar]
- Cunningham M. W., Russell S. M. Study of heart-reactive antibody in antisera and hybridoma culture fluids against group A streptococci. Infect Immun. 1983 Nov;42(2):531–538. doi: 10.1128/iai.42.2.531-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Sequence of myosin-crossreactive epitopes of streptococcal M protein. J Exp Med. 1986 Nov 1;164(5):1785–1790. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenderson P. G., Fischetti V. A., Cunningham M. W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989 Apr 1;142(7):2475–2481. [PubMed] [Google Scholar]
- Galán J. E., Timoney J. F. Molecular analysis of the M protein of Streptococcus equi and cloning and expression of the M protein gene in Escherichia coli. Infect Immun. 1987 Dec;55(12):3181–3187. doi: 10.1128/iai.55.12.3181-3187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- KAPLAN M. H., BOLANDE R., RAKITA L., BLAIR J. PRESENCE OF BOUND IMMUNOGLOBULINS AND COMPLEMENT IN THE MYOCARDIUM IN ACUTE RHEUMATIC FEVER. ASSOCIATION WITH CARDIAC FAILURE. N Engl J Med. 1964 Sep 24;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. I. PROPERTIES OF AN ANTIGEN IN CERTAIN STRAINS OF GROUP A STREPTOCOCCI EXHIBITING AN IMMUNOLOGIC CROSS-REACTION WITH HUMAN HEART TISSUE. J Immunol. 1963 Apr;90:595–606. [PubMed] [Google Scholar]
- Krisher K., Cunningham M. W. Myosin: a link between streptococci and heart. Science. 1985 Jan 25;227(4685):413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem Biophys Res Commun. 1986 Oct 30;140(2):684–690. doi: 10.1016/0006-291x(86)90786-2. [DOI] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Trus B. L., Fischetti V. A. Presence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological properties. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1064–1068. doi: 10.1073/pnas.82.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. M., Crossley C. A., Ayoub E. M., Harley J. B., Cunningham M. W. Poststreptococcal anti-myosin antibody idiotype associated with systemic lupus erythematosus and Sjögren's syndrome. J Infect Dis. 1993 Oct;168(4):915–921. doi: 10.1093/infdis/168.4.915. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Hollingshead S. K., Fischetti V. A. Homologous regions within M protein genes in group A streptococci of different serotypes. Infect Immun. 1986 May;52(2):609–612. doi: 10.1128/iai.52.2.609-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M., Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993 Mar;57(1):109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. C., Fox A., Fox K., Addy C., Garrison C. Z., Herron B., Brunson C., Betcher G. Role of group C beta-hemolytic streptococci in pharyngitis: epidemiologic study of clinical features associated with isolation of group C streptococci. J Clin Microbiol. 1993 Apr;31(4):808–811. doi: 10.1128/jcm.31.4.808-811.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasuk G. P., Inouye S., Ito H., Itakura K., Inouye M. Effects of the complete removal of basic amino acid residues from the signal peptide on secretion of lipoprotein in Escherichia coli. J Biol Chem. 1983 Jun 10;258(11):7141–7148. [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Pinney A. M. An M-associated protein antigen (MAP) of group A streptococci. J Hyg (Lond) 1971 Dec;69(4):553–564. doi: 10.1017/s0022172400021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yu C. E., Ferretti J. J. Molecular epidemiologic analysis of the type A streptococcal exotoxin (erythrogenic toxin) gene (speA) in clinical Streptococcus pyogenes strains. Infect Immun. 1989 Dec;57(12):3715–3719. doi: 10.1128/iai.57.12.3715-3719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Freimer E. H. An immunological relationship between the group. A streptococcus and mammalian muscle. J Exp Med. 1966 Oct 1;124(4):661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]