Abstract
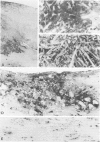
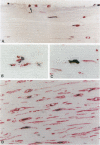
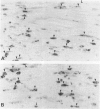
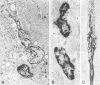
Death of intimal tissue may lead to plaque rupture with thrombosis, which is the basis of the most severe clinical consequences of atherosclerosis. Little is known about the mechanisms that promote intimal cell death or its nature. This work was undertaken to elucidate the extent to which, the cell types in which, and where programmed cell death, apoptosis, might occur in atherosclerotic lesions. The material was fibrous or fibro-fatty non-ulcerated lesions from the human thoracic aorta and coronary arteries. Apoptosis was indicated by the in situ labeling of internucleosomally degraded DNA with the TUNEL technique, which has a preference for apoptosis as compared with cell necrosis and was combined with the immunohistochemical typing of cells. Apoptosis was corroborated by morphological criteria on the light and electron microscope levels and by the presence of an apoptosis-specific protein. It was common in the lesions and virtually absent in non-atherosclerotic regions. It occurred in smooth muscle cells subendothelially, in places of the fibrous cap, and in the underlying media, which may destabilize the plaque and promote rupture. Inflammatory cells, ie, macrophages and T cells, appeared abundantly subendothelially, in the fibrous cap, and in the shoulder regions, and apoptosis was common, maybe reflecting a means for quenching of the inflammatory reaction. Many macrophages contained abundant apoptotic material indicative of phagocytosis of apoptotic cells, but the occurrence of apoptosis, even in some of these cells, and of apoptotic material extracellularly and the very high numbers of apoptotic cells that were encountered may indicate insufficient mechanisms for the removal of apoptotic cells in the atherosclerotic lesion. It is not possible to decide as yet whether this is due to overloading with cellular material by inflammation and cell multiplication, to an increased frequency of apoptosis, to a reduction of the removal/degradation of apoptotic material by macrophages, or a combination of these factors.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Allen P. D., Bustin S. A., Newland A. C. The role of apoptosis (programmed cell death) in haemopoiesis and the immune system. Blood Rev. 1993 Mar;7(1):63–73. doi: 10.1016/0268-960x(93)90025-y. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Newby A. C. Deregulated expression of the c-myc oncogene abolishes inhibition of proliferation of rat vascular smooth muscle cells by serum reduction, interferon-gamma, heparin, and cyclic nucleotide analogues and induces apoptosis. Circ Res. 1994 Mar;74(3):525–536. doi: 10.1161/01.res.74.3.525. [DOI] [PubMed] [Google Scholar]
- Björkerud S., Björkerud B., Joelsson M. Structural organization of reconstituted human arterial smooth muscle tissue. Arterioscler Thromb. 1994 Apr;14(4):644–651. doi: 10.1161/01.atv.14.4.644. [DOI] [PubMed] [Google Scholar]
- Brown D. L., Hibbs M. S., Kearney M., Loushin C., Isner J. M. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995 Apr 15;91(8):2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Cheng G. C., Loree H. M., Kamm R. D., Fishbein M. C., Lee R. T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993 Apr;87(4):1179–1187. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Richardson P. D., Woolf N., Katz D. R., Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993 May;69(5):377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Thomas A. C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985 Apr;53(4):363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V. A., Savill J. S., Haslett C., Bratton D. L., Doherty D. E., Campbell P. A., Henson P. M. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992 Dec 15;149(12):4029–4035. [PubMed] [Google Scholar]
- Falk E., Shah P. K., Fuster V. Coronary plaque disruption. Circulation. 1995 Aug 1;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992 Jan 30;326(5):310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Kranzhöfer R., Clark S., Libby P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):402–406. doi: 10.1073/pnas.92.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995 Jul;9(10):974–980. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. J., Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995 Aug;147(2):251–266. [PMC free article] [PubMed] [Google Scholar]
- Gold R., Schmied M., Giegerich G., Breitschopf H., Hartung H. P., Toyka K. V., Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994 Aug;71(2):219–225. [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- Grand R. J., Milner A. E., Mustoe T., Johnson G. D., Owen D., Grant M. L., Gregory C. D. A novel protein expressed in mammalian cells undergoing apoptosis. Exp Cell Res. 1995 Jun;218(2):439–451. doi: 10.1006/excr.1995.1177. [DOI] [PubMed] [Google Scholar]
- Han D. K., Haudenschild C. C., Hong M. K., Tinkle B. T., Leon M. B., Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol. 1995 Aug;147(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- Haraoka S., Shimokama T., Watanabe T. Participation of T lymphocytes in atherogenesis: sequential and quantitative observation of aortic lesions of rats with diet-induced hypercholesterolaemia using en face double immunostaining. Virchows Arch. 1995;426(3):307–315. doi: 10.1007/BF00191369. [DOI] [PubMed] [Google Scholar]
- Haslett C., Savill J. S., Whyte M. K., Stern M., Dransfield I., Meagher L. C. Granulocyte apoptosis and the control of inflammation. Philos Trans R Soc Lond B Biol Sci. 1994 Aug 30;345(1313):327–333. doi: 10.1098/rstb.1994.0113. [DOI] [PubMed] [Google Scholar]
- Henney A. M., Wakeley P. R., Davies M. J., Foster K., Hembry R., Murphy G., Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraishi K., Suzuki K., Hakomori S., Adachi M. Le(y) antigen expression is correlated with apoptosis (programmed cell death). Glycobiology. 1993 Aug;3(4):381–390. doi: 10.1093/glycob/3.4.381. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Kearney M., Bortman S., Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995 Jun 1;91(11):2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., Cambier B. A., Bortier H. E., De Meyer G. R., Declercq S. C., van Cauwelaert P. A., Bultinck J. Foam cell replication and smooth muscle cell apoptosis in human saphenous vein grafts. Histopathology. 1994 Oct;25(4):365–371. doi: 10.1111/j.1365-2559.1994.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Kornbluth R. S. The immunological potential of apoptotic debris produced by tumor cells and during HIV infection. Immunol Lett. 1994 Dec;43(1-2):125–132. doi: 10.1016/0165-2478(94)00149-9. [DOI] [PubMed] [Google Scholar]
- Lendon C. L., Davies M. J., Born G. V., Richardson P. D. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991 Mar;87(1):87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- Lennon S. V., Martin S. J., Cotter T. G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991 Mar;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Leszczynski D., Zhao Y., Luokkamäki M., Foegh M. L. Apoptosis of vascular smooth muscle cells. Protein kinase C and oncoprotein Bcl-2 are involved in regulation of apoptosis in non-transformed rat vascular smooth muscle cells. Am J Pathol. 1994 Dec;145(6):1265–1270. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Moreno P. R., Falk E., Palacios I. F., Newell J. B., Fuster V., Fallon J. T. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994 Aug;90(2):775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- Munn D. H., Beall A. C., Song D., Wrenn R. W., Throckmorton D. C. Activation-induced apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon gamma. J Exp Med. 1995 Jan 1;181(1):127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Southgate K. M., Davies M. Extracellular matrix degrading metalloproteinases in the pathogenesis of arteriosclerosis. Basic Res Cardiol. 1994;89 (Suppl 1):59–70. doi: 10.1007/978-3-642-85660-0_6. [DOI] [PubMed] [Google Scholar]
- Nikkari S. T., O'Brien K. D., Ferguson M., Hatsukami T., Welgus H. G., Alpers C. E., Clowes A. W. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995 Sep 15;92(6):1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- Ottnad E., Parthasarathy S., Sambrano G. R., Ramprasad M. P., Quehenberger O., Kondratenko N., Green S., Steinberg D. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low density lipoprotein: partial purification and role in recognition of oxidatively damaged cells. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1391–1395. doi: 10.1073/pnas.92.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston R. N., Hussain I. F. The immunohistochemical heterogeneity of atheroma macrophages: comparison with lymphoid tissues suggests that recently blood-derived macrophages can be distinguished from longer-resident cells. J Histochem Cytochem. 1993 Oct;41(10):1503–1512. doi: 10.1177/41.10.7504008. [DOI] [PubMed] [Google Scholar]
- Reid V. C., Hardwick S. J., Mitchinson M. J. Fragmentation of DNA in P388D1 macrophages exposed to oxidised low-density lipoprotein. FEBS Lett. 1993 Oct 18;332(3):218–220. doi: 10.1016/0014-5793(93)80635-8. [DOI] [PubMed] [Google Scholar]
- Reid V. C., Mitchinson M. J., Skepper J. N. Cytotoxicity of oxidized low-density lipoprotein to mouse peritoneal macrophages: an ultrastructural study. J Pathol. 1993 Dec;171(4):321–328. doi: 10.1002/path.1711710413. [DOI] [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Sambrano G. R., Steinberg D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Apoptosis in disease. Eur J Clin Invest. 1994 Nov;24(11):715–723. doi: 10.1111/j.1365-2362.1994.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Schmied M., Breitschopf H., Gold R., Zischler H., Rothe G., Wekerle H., Lassmann H. Apoptosis of T lymphocytes in experimental autoimmune encephalomyelitis. Evidence for programmed cell death as a mechanism to control inflammation in the brain. Am J Pathol. 1993 Aug;143(2):446–452. [PMC free article] [PubMed] [Google Scholar]
- Shah P. K., Falk E., Badimon J. J., Fernandez-Ortiz A., Mailhac A., Villareal-Levy G., Fallon J. T., Regnstrom J., Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995 Sep 15;92(6):1565–1569. [PubMed] [Google Scholar]
- Stemme S., Faber B., Holm J., Wiklund O., Witztum J. L., Hansson G. K. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994 Jan;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- van der Wal A. C., Becker A. E., van der Loos C. M., Das P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994 Jan;89(1):36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]