Abstract
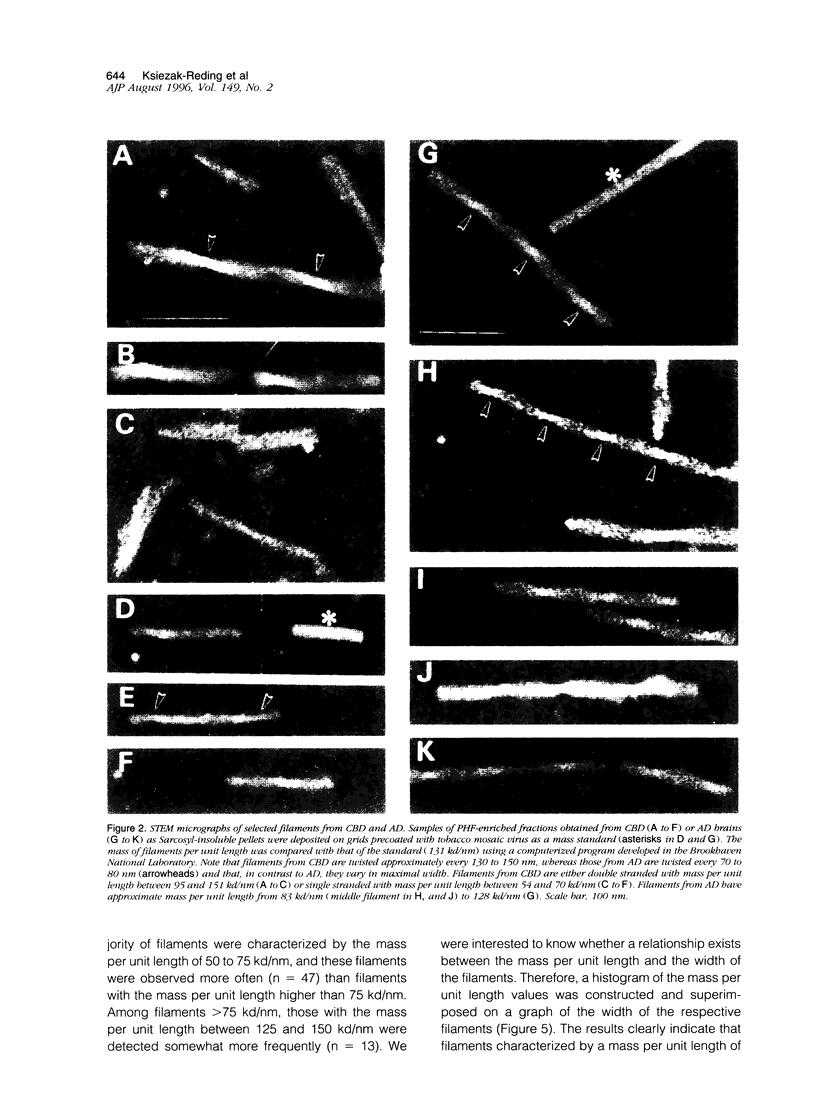
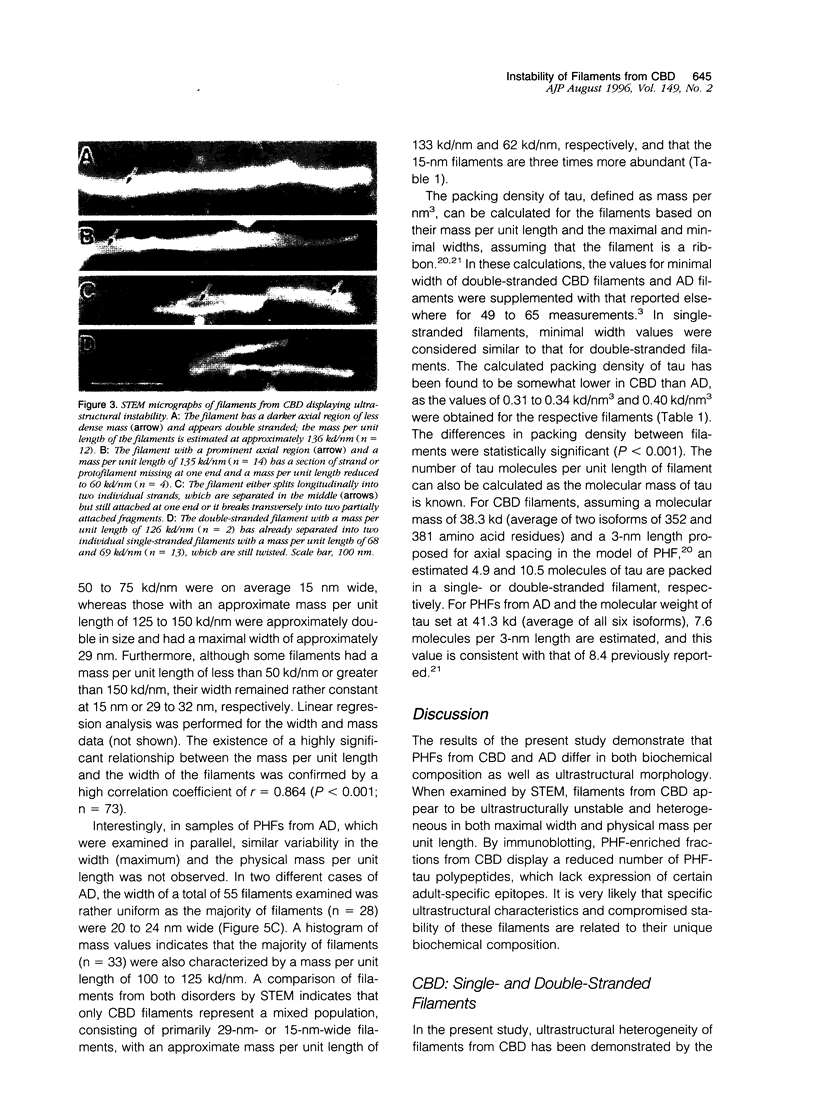
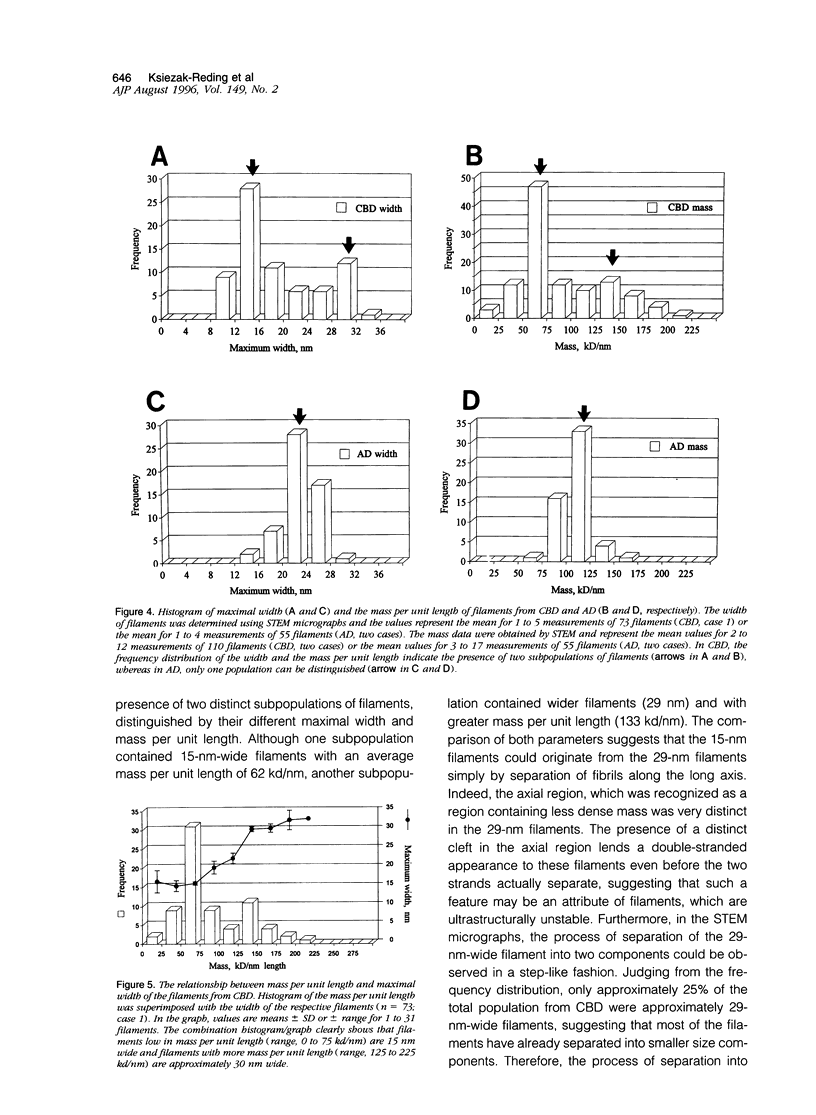
Paired helical filaments (PHFs) accumulate in the brains of subjects affected with Alzheimer's disease (AD) and certain other neurodegenerative disorders, including corticobasal degeneration (CBD). Electron microscope studies have shown that PHFs from CBD differ from those of AD by being wider and having a longer periodicity of the helical twist. Moreover, PHFs from CBD have been shown to be primarily composed of two rather than three highly phosphorylated polypeptides of tau (PHF-tau), with these polypeptides expressing no exons 3 and 10. To further explore the relationship between the heterogeneity of PHF-tau and the appearance of abnormal filaments, the ultrastructure and physical parameters such as mass per unit length and dimensions were compared in filaments from CBD and AD using high resolution scanning transmission electron microscopy (STEM). Filament-enriched fractions were isolated as Sarcosyl-insoluble pellets and for STEM studies, samples were freeze-dried without prior fixation or staining. Ultrastructurally, PHFs from CBD were shown to be a heterogeneous population as double- and single-stranded filaments could be identified based on their width and physical mass per unit length expressed in kilodaltons (kd) per nanometer (nm). Less abundant, double-stranded filaments had a maximal width of 29 nm and a mass per unit length of 133 kd/nm, whereas three times more abundant single-stranded filaments were 15 nm wide and bad a mass per unit length of 62 kd/nm. Double-stranded filaments also displayed a distinct axial region of less dense mass, which appeared to divide the PHFs into two protofilament-like strands. Furthermore, these filaments were frequently observed to physically separate along the long axis into two single strands or to break longitudinally. In contrast, PHFs from AD were ultrastructurally stable and uniform both in their width (22 nm) and physical mass per unit length (104 kd/nm). The ultrastructural features indicate that filaments of CBD and AD differ both in stability and packing of tau and that CBD filaments, composed of two distinct protofilaments, are more labile under STEM conditions. As fixed and stained filaments from CBD have been shown to be stable and uniform in size by conventional transmission electron microscopy, STEM studies may be particularly suitable for detecting instability of unstained and unfixed filaments. The results also suggest that molecular heterogeneity and/or post-translational modifications of tau may strongly influence the morphology and stability of abnormal filaments.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braak H., Braak E., Grundke-Iqbal I., Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986 Apr 24;65(3):351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- Butler M., Shelanski M. L. Microheterogeneity of microtubule-associated tau proteins is due to differences in phosphorylation. J Neurochem. 1986 Nov;47(5):1517–1522. doi: 10.1111/j.1471-4159.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2288–2292. doi: 10.1073/pnas.88.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A. Structural aspects of pathology in Alzheimer's disease. Biochim Biophys Acta. 1990 Nov 14;1096(1):1–9. doi: 10.1016/0925-4439(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Wischik C. M. Image reconstruction of the Alzheimer paired helical filament. EMBO J. 1985 Dec 30;4(13B):3661–3665. doi: 10.1002/j.1460-2075.1985.tb04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Dickson D. W. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995 Jun;146(6):1388–1396. [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Ksiezak-Reding H., Liu W. K., Vincent I., Yen S. H., Dickson D. W. Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol. 1995;90(1):37–43. doi: 10.1007/BF00294457. [DOI] [PubMed] [Google Scholar]
- Flament S., Delacourte A., Verny M., Hauw J. J., Javoy-Agid F. Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol. 1991;81(6):591–596. doi: 10.1007/BF00296367. [DOI] [PubMed] [Google Scholar]
- Furcinitti P. S., van Oostrum J., Burnett R. M. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 1989 Dec 1;8(12):3563–3570. doi: 10.1002/j.1460-2075.1989.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990 Dec;9(13):4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992 Jan;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Potier M. C., Ulrich J., Crowther R. A. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989 Feb;8(2):393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P., Schein J. D., Binder L. I. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992 Jan 5;267(1):564–569. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw J. J., Verny M., Delaère P., Cervera P., He Y., Duyckaerts C. Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer's disease and aging. Neurosci Lett. 1990 Nov 13;119(2):182–186. doi: 10.1016/0304-3940(90)90829-x. [DOI] [PubMed] [Google Scholar]
- Hof P. R., Bouras C., Perl D. P., Morrison J. H. Quantitative neuropathologic analysis of Pick's disease cases: cortical distribution of Pick bodies and coexistence with Alzheimer's disease. Acta Neuropathol. 1994;87(2):115–124. doi: 10.1007/BF00296179. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kato S., Nakamura H. Presence of two different fibril subtypes in the Pick body: an immunoelectron microscopic study. Acta Neuropathol. 1990;81(2):125–129. doi: 10.1007/BF00334500. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Bakalis S., Neve R. L. Developmentally regulated expression of specific tau sequences. Neuron. 1989 Apr;2(4):1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Leibowitz R. L., Bowser R., Davies P. Binding of Alz 50 depends on Phe8 in tau synthetic peptides and varies between native and denatured tau proteins. Brain Res. 1995 Oct 30;697(1-2):63–75. doi: 10.1016/0006-8993(95)00785-o. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Liu W. K., Yen S. H. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992 Dec 4;597(2):209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Morgan K., Dickson D. W. Tau immunoreactivity and SDS solubility of two populations of paired helical filaments that differ in morphology. Brain Res. 1994 Jun 27;649(1-2):185–196. doi: 10.1016/0006-8993(94)91063-4. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Morgan K., Mattiace L. A., Davies P., Liu W. K., Yen S. H., Weidenheim K., Dickson D. W. Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am J Pathol. 1994 Dec;145(6):1496–1508. [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Shafit-Zagardo B., Yen S. H. Differential expression of exons 10 and 11 in normal tau and tau associated with paired helical filaments. J Neurosci Res. 1995 Aug 1;41(5):583–593. doi: 10.1002/jnr.490410504. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Wall J. S. Mass and physical dimensions of two distinct populations of paired helical filaments. Neurobiol Aging. 1994 Jan-Feb;15(1):11–19. doi: 10.1016/0197-4580(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Masliah E., Hansen L. A., Quijada S., DeTeresa R., Alford M., Kauss J., Terry R. Late onset dementia with argyrophilic grains and subcortical tangles or atypical progressive supranuclear palsy? Ann Neurol. 1991 Apr;29(4):389–396. doi: 10.1002/ana.410290409. [DOI] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994 Oct;13(4):989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Van de Voorde A., Martin J. J., Gheuens J. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84(3):265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]
- Metuzals J., Robitaille Y., Houghton S., Gauthier S., Kang C. Y., Leblanc R. Neuronal transformations in Alzheimer's disease. Cell Tissue Res. 1988 May;252(2):239–248. doi: 10.1007/BF00214366. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Katsuragi S., Araki K., Hashimura T., Kimura T., Kuramoto R. Ultrastructure of neurofibrillary tangles in Alzheimer's disease. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(4):267–273. doi: 10.1007/BF02899091. [DOI] [PubMed] [Google Scholar]
- Mori H., Nishimura M., Namba Y., Oda M. Corticobasal degeneration: a disease with widespread appearance of abnormal tau and neurofibrillary tangles, and its relation to progressive supranuclear palsy. Acta Neuropathol. 1994;88(2):113–121. doi: 10.1007/BF00294503. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Titani K., Ihara Y. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron. 1993 Jun;10(6):1151–1160. doi: 10.1016/0896-6273(93)90063-w. [DOI] [PubMed] [Google Scholar]
- Murayama S., Mori H., Ihara Y., Tomonaga M. Immunocytochemical and ultrastructural studies of Pick's disease. Ann Neurol. 1990 Apr;27(4):394–405. doi: 10.1002/ana.410270407. [DOI] [PubMed] [Google Scholar]
- Otvos L., Jr, Feiner L., Lang E., Szendrei G. I., Goedert M., Lee V. M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994 Dec 15;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Oyanagi K., Takahashi H., Wakabayashi K., Ikuta F. Large neurons in the neostriatum in Alzheimer's disease and progressive supranuclear palsy: a topographic, histologic and ultrastructural investigation. Brain Res. 1991 Mar 29;544(2):221–226. doi: 10.1016/0006-8993(91)90057-3. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type: II. Electron microscopy and pathogenetic implications. Effects of fixation on the morphology of the Alzheimer's abnormal filaments. Lab Invest. 1989 Mar;60(3):375–389. [PubMed] [Google Scholar]
- Paulus W., Selim M. Corticonigral degeneration with neuronal achromasia and basal neurofibrillary tangles. Acta Neuropathol. 1990;81(1):89–94. doi: 10.1007/BF00662643. [DOI] [PubMed] [Google Scholar]
- Perry G., Stewart D., Friedman R., Manetto V., Autilio-Gambetti L., Gambetti P. Filaments of Pick's bodies contain altered cytoskeletal elements. Am J Pathol. 1987 Jun;127(3):559–568. [PMC free article] [PubMed] [Google Scholar]
- Pollanen M. S., Markiewicz P., Bergeron C., Goh M. C. Twisted ribbon structure of paired helical filaments revealed by atomic force microscopy. Am J Pathol. 1994 May;144(5):869–873. [PMC free article] [PubMed] [Google Scholar]
- Pollock N. J., Mirra S. S., Binder L. I., Hansen L. A., Wood J. G. Filamentous aggregates in Pick's disease, progressive supranuclear palsy, and Alzheimer's disease share antigenic determinants with microtubule-associated protein, tau. Lancet. 1986 Nov 22;2(8517):1211–1211. doi: 10.1016/s0140-6736(86)92212-9. [DOI] [PubMed] [Google Scholar]
- Ruben G. C., Novak M., Edwards P. C., Iqbal K. Alzheimer paired helical filaments, untreated and pronase digested, studied by vertical platinum-carbon replication and high resolution transmission electron microscopy. Brain Res. 1995 Mar 27;675(1-2):1–12. doi: 10.1016/0006-8993(94)01437-m. [DOI] [PubMed] [Google Scholar]
- Sparkman D. R., Johnson S. A., Hammon K. M., Allison P. M., White C. L., 3rd Isolation of the insoluble straight fibrils of Pick's disease. J Neurol Sci. 1987 Sep;80(2-3):173–184. doi: 10.1016/0022-510x(87)90153-5. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Whitehouse P. J., Perry G., Davies P., Autilio-Gambetti L., Gambetti P. Alz 50 recognizes abnormal filaments in Alzheimer's disease and progressive supranuclear palsy. Ann Neurol. 1988 Sep;24(3):407–413. doi: 10.1002/ana.410240309. [DOI] [PubMed] [Google Scholar]
- Takauchi S., Hosomi M., Marasigan S., Sato M., Hayashi S., Miyoshi K. An ultrastructural study of Pick bodies. Acta Neuropathol. 1984;64(4):344–348. doi: 10.1007/BF00690400. [DOI] [PubMed] [Google Scholar]
- Tokutake S., Oyanagi S. Mechanical instability of Pick bodies and their isolation in an intact form using urea solution. Neurosci Lett. 1993 Nov 26;163(1):15–18. doi: 10.1016/0304-3940(93)90218-a. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Oyanagi K., Makifuchi T., Ikuta F., Homma A., Homma Y., Horikawa Y., Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. 1994;87(6):545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- Wall J. S., Hainfeld J. F. Mass mapping with the scanning transmission electron microscope. Annu Rev Biophys Biophys Chem. 1986;15:355–376. doi: 10.1146/annurev.bb.15.060186.002035. [DOI] [PubMed] [Google Scholar]
- Wiche G., Oberkanins C., Himmler A. Molecular structure and function of microtubule-associated proteins. Int Rev Cytol. 1991;124:217–273. doi: 10.1016/s0074-7696(08)61528-4. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Crowther R. A., Stewart M., Roth M. Subunit structure of paired helical filaments in Alzheimer's disease. J Cell Biol. 1985 Jun;100(6):1905–1912. doi: 10.1083/jcb.100.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. M., Wen G. Y. Substructures of paired helical filaments from Alzheimer's disease neurofibrillary tangles. Acta Neuropathol. 1985;66(2):173–176. doi: 10.1007/BF00688696. [DOI] [PubMed] [Google Scholar]