Abstract
Little is known about alterations in cell cycle regulatory proteins such as p53 and WAF1 in diffuse alveolar damage (DAD). We hypothesized that up-regulation of p53 and WAF1 in type II pneumocytes in DAD is associated with underlying DNA damage and apoptosis. Twenty cases of DAD and twenty control specimens of lung adjacent to resected tumors were studied. Immunohistochemical stains with antibodies recognizing p53 and WAF1 were performed, and apoptosis was assessed in sixteen cases by the nick end-labeling method. We identified p53 expression and apoptosis in all cases of DAD but not in any of the control lungs. We detected WAF1 expression in nineteen of twenty cases of DAD and in sixteen of twenty control lungs. In general, the distribution and intensity of WAF1 staining were greater in DAD than in control lungs. Staining for both p53 and WAF1 and labeling of apoptotic cells in DAD were usually focal ( < 10% of cells) and predominantly localized in type II pneumocytes. We conclude that increased p53 and WAF1 expression in DAD reflects normal physiological up-regulation in response to cellular and DNA damage and is associated with apoptosis of type II pneumocytes. p53-dependent apoptosis may contribute to the pathogenesis of this disease.
Full text
PDF

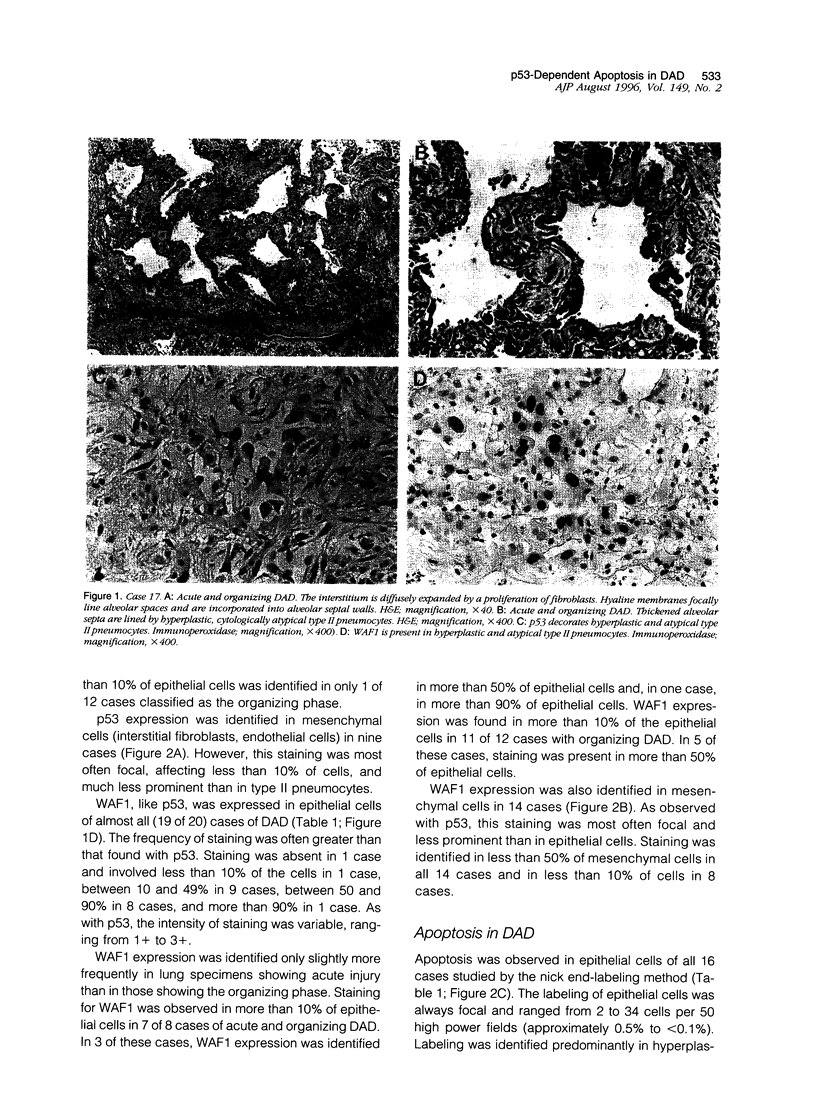
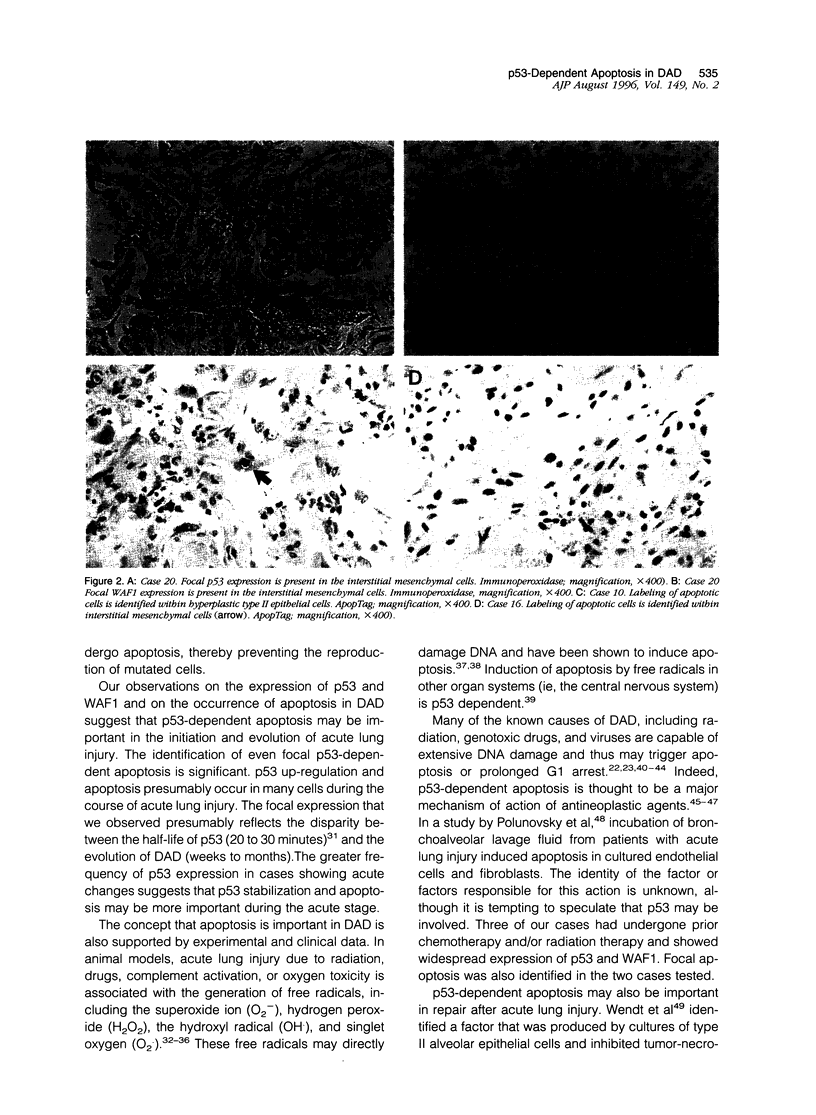



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashbaugh D. G., Bigelow D. B., Petty T. L., Levine B. E. Acute respiratory distress in adults. Lancet. 1967 Aug 12;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Basic pattern of tissue repair in human lungs following unspecific injury. Chest. 1974 Apr;65(Suppl):14S–19S. doi: 10.1378/chest.65.4_supplement.14s. [DOI] [PubMed] [Google Scholar]
- Bertrand Y. Oxygen-free radicals and lipid peroxidation in adult respiratory distress syndrome. Intensive Care Med. 1985;11(2):56–60. doi: 10.1007/BF00254774. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Polunovsky V. A., Ingbar D. H. Repair after acute lung injury. Chest. 1994 Mar;105(3 Suppl):118S–121S. doi: 10.1378/chest.105.3_supplement.118s. [DOI] [PubMed] [Google Scholar]
- Blaisdell F. W., Schlobohm R. M. The respiratory distress syndrome: a review. Surgery. 1973 Aug;74(2):251–262. [PubMed] [Google Scholar]
- Bodner S. M., Minna J. D., Jensen S. M., D'Amico D., Carbone D., Mitsudomi T., Fedorko J., Buchhagen D. L., Nau M. M., Gazdar A. F. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992 Apr;7(4):743–749. [PubMed] [Google Scholar]
- Braun S. R., doPico A., Olson C. E., Caldwell W. Low-dose radiation pneumonitis. Cancer. 1975 May;35(5):1322–1324. doi: 10.1002/1097-0142(197505)35:5<1322::aid-cncr2820350512>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cagle P. T., Brown R. W., Lebovitz R. M. p53 immunostaining in the differentiation of reactive processes from malignancy in pleural biopsy specimens. Hum Pathol. 1994 May;25(5):443–448. doi: 10.1016/0046-8177(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Collins J. F., Smith J. D., Coalson J. J., Johanson W. G., Jr Variability in lung collagen amounts after prolonged support of acute respiratory failure. Chest. 1984 May;85(5):641–646. doi: 10.1378/chest.85.5.641. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Jr, White D. A., Matthay R. A. Drug-induced pulmonary disease. Part 1: Cytotoxic drugs. Am Rev Respir Dis. 1986 Feb;133(2):321–340. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Normobaric oxygen toxicity of the lung. N Engl J Med. 1980 Jul 10;303(2):76–86. doi: 10.1056/NEJM198007103030204. [DOI] [PubMed] [Google Scholar]
- Di Leonardo A., Linke S. P., Clarkin K., Wahl G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994 Nov 1;8(21):2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Frank L., Massaro D. Oxygen toxicity. Am J Med. 1980 Jul;69(1):117–126. doi: 10.1016/0002-9343(80)90509-4. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Ishizaki M., Masuda Y., Kimura G., Kawanami O., Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol. 1987 Jan;126(1):171–182. [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Tosco R., Wheelis R. F., Gould N. S., Kapanci Y. Oxygen pneumonitis in man. Ultrastructural observations on the development of alveolar lesions. Lab Invest. 1972 May;26(5):499–508. [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Greene R. Adult respiratory distress syndrome: acute alveolar damage. Radiology. 1987 Apr;163(1):57–66. doi: 10.1148/radiology.163.1.3823457. [DOI] [PubMed] [Google Scholar]
- Gross N. J. The pathogenesis of radiation-induced lung damage. Lung. 1981;159(3):115–125. doi: 10.1007/BF02713907. [DOI] [PubMed] [Google Scholar]
- Guinee D. G., Jr, Travis W. D., Trivers G. E., De Benedetti V. M., Cawley H., Welsh J. A., Bennett W. P., Jett J., Colby T. V., Tazelaar H. Gender comparisons in human lung cancer: analysis of p53 mutations, anti-p53 serum antibodies and C-erbB-2 expression. Carcinogenesis. 1995 May;16(5):993–1002. doi: 10.1093/carcin/16.5.993. [DOI] [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993 Oct 28;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Jiang H., Lin J., Su Z. Z., Collart F. R., Huberman E., Fisher P. B. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994 Nov;9(11):3397–3406. [PubMed] [Google Scholar]
- Kallakury B. V., Figge J., Ross J. S., Fisher H. A., Figge H. L., Jennings T. A. Association of p53 immunoreactivity with high gleason tumor grade in prostatic adenocarcinoma. Hum Pathol. 1994 Jan;25(1):92–97. doi: 10.1016/0046-8177(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Tosco R., Eggermann J., Gould V. E. Oxygen pneumonitis in man., Light- and electron-microscopic morphometric studies. Chest. 1972 Aug;62(2):162–169. doi: 10.1378/chest.62.2.162. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L., Bloor C. M., Leibow A. A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976 Oct;85(1):209–228. [PMC free article] [PubMed] [Google Scholar]
- Katzenstein A. L., Myers J. L., Mazur M. T. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol. 1986 Apr;10(4):256–267. [PubMed] [Google Scholar]
- Katzenstein A. L. Pathogenesis of "fibrosis" in interstitial pneumonia: an electron microscopic study. Hum Pathol. 1985 Oct;16(10):1015–1024. doi: 10.1016/s0046-8177(85)80279-3. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Jackman J., O'Connor P. M. Cell cycle control and cancer chemotherapy. J Cell Biochem. 1994 Apr;54(4):440–452. doi: 10.1002/jcb.240540411. [DOI] [PubMed] [Google Scholar]
- Krishna M., Woda B., Savas L., Baker S., Banner B. Expression of p53 antigen in inflamed and regenerated mucosa in ulcerative colitis and Crohn's disease. Mod Pathol. 1995 Aug;8(6):654–657. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Maity A., McKenna W. G., Muschel R. J. The molecular basis for cell cycle delays following ionizing radiation: a review. Radiother Oncol. 1994 Apr;31(1):1–13. doi: 10.1016/0167-8140(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Martin A. M., Jr, Soloway H. B., Simmons R. L. Pathologic anatomy of the lungs following shock and trauma. J Trauma. 1968 Sep;8(5):687–699. doi: 10.1097/00005373-196809000-00007. [DOI] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Murphy P. G., Myers D. S., Webster N. R., Jones J. G., Davies M. J. Direct detection of free radical generation in an in vivo model of acute lung injury. Free Radic Res Commun. 1991;15(3):167–176. doi: 10.3109/10715769109049137. [DOI] [PubMed] [Google Scholar]
- Murray J. F., Matthay M. A., Luce J. M., Flick M. R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988 Sep;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Tierney D. F. Biochemical and metabolic changes in the lung with oxygen, ozone, and nitrogen dioxide toxicity. Am Rev Respir Dis. 1978 Dec;118(6):1061–1090. doi: 10.1164/arrd.1978.118.6.1061. [DOI] [PubMed] [Google Scholar]
- Nash G., Blennerhassett J. B., Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artifical ventilation. N Engl J Med. 1967 Feb 16;276(7):368–374. doi: 10.1056/NEJM196702162760702. [DOI] [PubMed] [Google Scholar]
- Nash G., Foley F. D., Langlinais P. C. Pulmonary interstitial edema and hyaline membranes in adult burn patients. Electron microscopic observations. Hum Pathol. 1974 Mar;5(2):149–160. doi: 10.1016/s0046-8177(74)80062-6. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M., Kohn K. W. A fundamental role for cell cycle regulation in the chemosensitivity of cancer cells? Semin Cancer Biol. 1992 Dec;3(6):409–416. [PubMed] [Google Scholar]
- Polunovsky V. A., Chen B., Henke C., Snover D., Wendt C., Ingbar D. H., Bitterman P. B. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–397. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramael M., Lemmens G., Eerdekens C., Buysse C., Deblier I., Jacobs W., van Marck E. Immunoreactivity for p53 protein in malignant mesothelioma and non-neoplastic mesothelium. J Pathol. 1992 Dec;168(4):371–375. doi: 10.1002/path.1711680406. [DOI] [PubMed] [Google Scholar]
- Sheikh M. S., Li X. S., Chen J. C., Shao Z. M., Ordonez J. V., Fontana J. A. Mechanisms of regulation of WAF1/Cip1 gene expression in human breast carcinoma: role of p53-dependent and independent signal transduction pathways. Oncogene. 1994 Dec;9(12):3407–3415. [PubMed] [Google Scholar]
- Till G. O., Friedl H. P., Ward P. A. Lung injury and complement activation: role of neutrophils and xanthine oxidase. Free Radic Biol Med. 1991;10(6):379–386. doi: 10.1016/0891-5849(91)90046-6. [DOI] [PubMed] [Google Scholar]
- Trask C. W., Joannides T., Harper P. G., Tobias J. S., Spiro S. G., Geddes D. M., Souhami R. L., Beverly P. C. Radiation-induced lung fibrosis after treatment of small cell carcinoma of the lung with very high-dose cyclophosphamide. Cancer. 1985 Jan 1;55(1):57–60. doi: 10.1002/1097-0142(19850101)55:1<57::aid-cncr2820550110>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Villuendas R., Piris M. A., Orradre J. L., Mollejo M., Algara P., Sanchez L., Martinez J. C., Martinez P. P53 protein expression in lymphomas and reactive lymphoid tissue. J Pathol. 1992 Mar;166(3):235–241. doi: 10.1002/path.1711660305. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wendt C. H., Polunovsky V. A., Peterson M. S., Bitterman P. B., Ingbar D. H. Alveolar epithelial cells regulate the induction of endothelial cell apoptosis. Am J Physiol. 1994 Oct;267(4 Pt 1):C893–C900. doi: 10.1152/ajpcell.1994.267.4.C893. [DOI] [PubMed] [Google Scholar]
- Wood K. A., Youle R. J. Apoptosis and free radicals. Ann N Y Acad Sci. 1994 Nov 17;738:400–407. doi: 10.1111/j.1749-6632.1994.tb21829.x. [DOI] [PubMed] [Google Scholar]
- Wood K. A., Youle R. J. The role of free radicals and p53 in neuron apoptosis in vivo. J Neurosci. 1995 Aug;15(8):5851–5857. doi: 10.1523/JNEUROSCI.15-08-05851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]




