Abstract
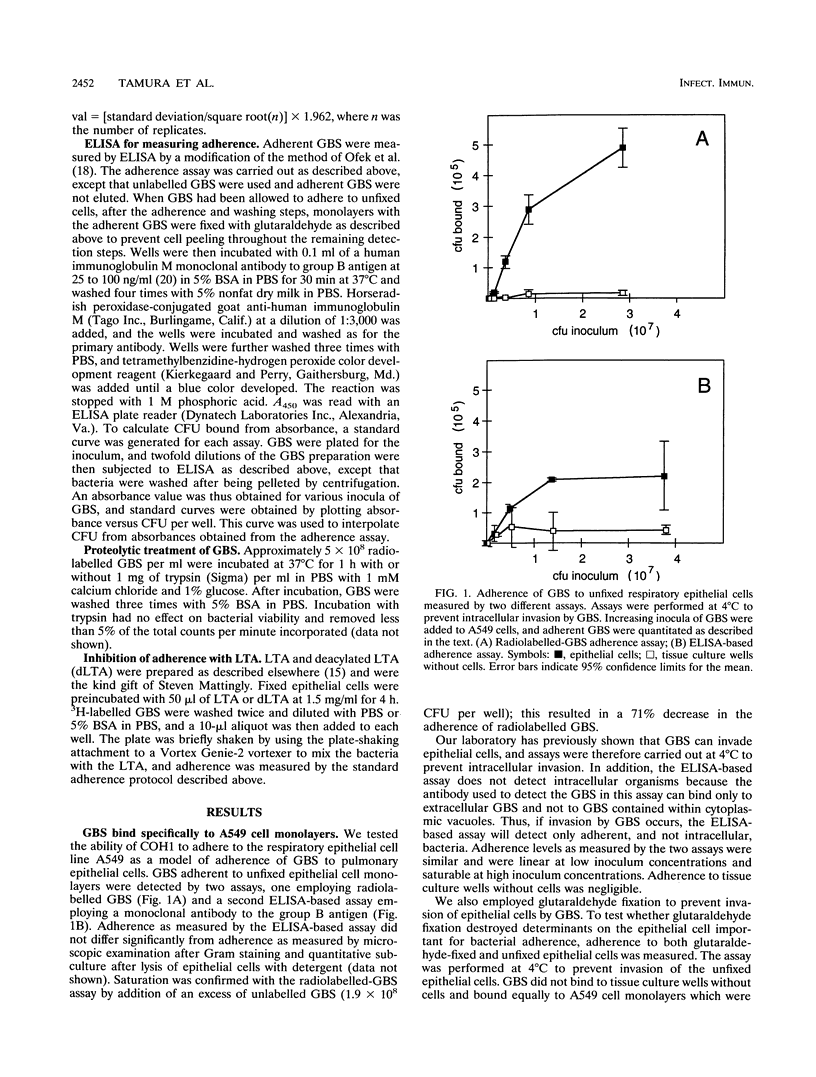
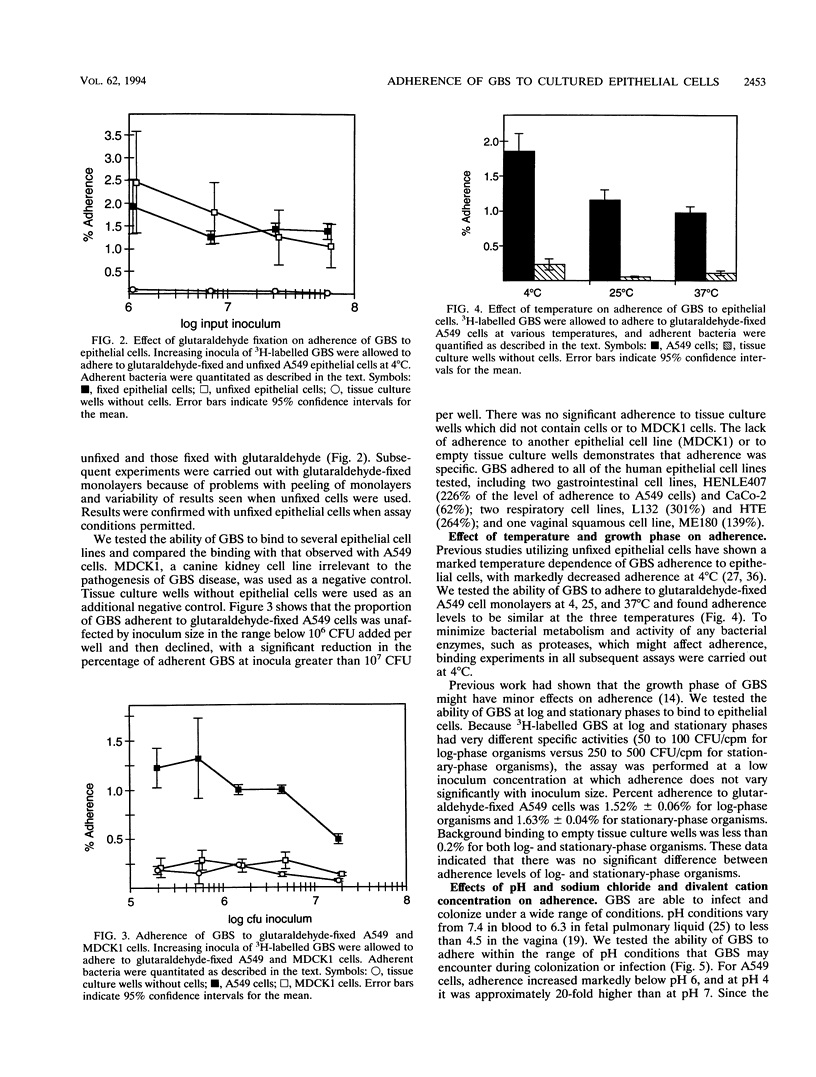
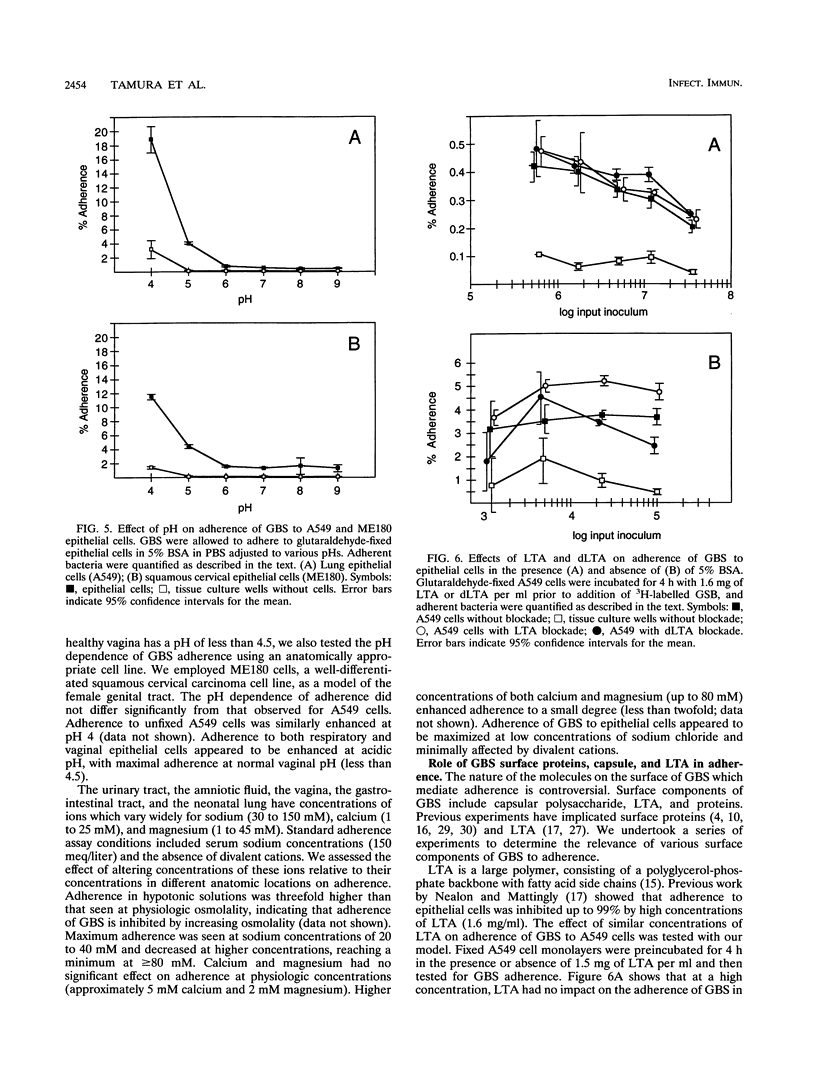
Group B streptococci (GBS) are the major cause of neonatal pneumonia, sepsis, and meningitis. Steps considered to be important in the pathogenesis of this infection include colonization of the rectum and vagina of the mother, aspiration of GBS into the fetal lung during or just prior to delivery, and invasion of GBS into pulmonary epithelial cells. We have previously demonstrated that GBS can invade pulmonary epithelial cells both in vivo and in vitro. Adherence of GBS to epithelial cells may play an important role in colonization of the rectum and vagina and constitute a first step in invasion of pulmonary epithelial cells. Because GBS can both adhere to and invade epithelial cells, we have developed two assays for GBS adherence which measure cell surface and not intracellular bacteria. Using these assays, we were able to demonstrate specific adherence of GBS to pulmonary epithelial cells. Adherence levels were similar at 4 and 37 degrees C and for log- and stationary-phase bacteria. Physiologic conditions vary considerably between the rectum, vagina, and lung, and a range of conditions was therefore tested. Adherence was enhanced in hypotonic solutions, while magnesium and calcium had no effect on adherence at physiologic concentrations. In comparison with adherence at neutral pH, adherence was increased 6- to 20-fold at pH 4, which is the normal vaginal pH. Neither capsular polysaccharide nor lipoteichoic acid was important for adherence in these assays. Treatment of GBS with trypsin decreased their adherence by more than 75%, indicating that surface proteins play an important role.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botta G. A. Hormonal and type-dependent adhesion of group B streptococci to human vaginal cells. Infect Immun. 1979 Sep;25(3):1084–1086. doi: 10.1128/iai.25.3.1084-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton R. A., Baker C. J. Role of adherence in the pathogenesis of neonatal group B streptococcal infection. Infect Immun. 1983 Feb;39(2):837–843. doi: 10.1128/iai.39.2.837-843.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova T. N., Grabovskaya K. B., Rýc M., Jelínková J. The adhesin structures involved in the adherence of group B streptococci to human vaginal cells. Folia Microbiol (Praha) 1986;31(5):394–401. doi: 10.1007/BF02936605. [DOI] [PubMed] [Google Scholar]
- Butler K. M., Baker C. J., Edwards M. S. Interaction of soluble fibronectin with group B streptococci. Infect Immun. 1987 Oct;55(10):2404–2408. doi: 10.1128/iai.55.10.2404-2408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F., Taylor L. Adherence of group B streptococci to buccal epithelial cells in neonates with different gestational ages. J Perinat Med. 1990;18(6):455–458. doi: 10.1515/jpme.1990.18.6.455. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Calderwood S. B., Donohue-Rolfe A., Keusch G. T., Kaper J. B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990 Jun;58(6):1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991 Jun 28;65(7):1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- Gibson R. L., Lee M. K., Soderland C., Chi E. Y., Rubens C. E. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun. 1993 Feb;61(2):478–485. doi: 10.1128/iai.61.2.478-485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt J. C., Jr, Panos C. Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture. Infect Immun. 1984 Feb;43(2):670–677. doi: 10.1128/iai.43.2.670-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmig R., Halaburt J. T., Uldbjert N., Thomsen A. C., Stenderup A. Increased cell adherence of group B streptococci from preterm infants with neonatal sepsis. Obstet Gynecol. 1990 Nov;76(5 Pt 1):825–827. doi: 10.1097/00006250-199011000-00020. [DOI] [PubMed] [Google Scholar]
- Jelínková J., Grabovskaya K. B., Rýc M., Bulgakova T. N., Totolian A. A. Adherence of vaginal and pharyngeal strains of group B streptococci to human vaginal and pharyngeal epithelial cells. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Nov;262(4):492–499. doi: 10.1016/s0176-6724(86)80143-2. [DOI] [PubMed] [Google Scholar]
- Kubín V., Rýc M. Adherence of group B streptococci to human buccal epithelial cells, its dependence on the biological state of the culture. Folia Microbiol (Praha) 1988;33(3):224–229. doi: 10.1007/BF02925909. [DOI] [PubMed] [Google Scholar]
- Maurer J. J., Mattingly S. J. Molecular analysis of lipoteichoic acid from Streptococcus agalactiae. J Bacteriol. 1991 Jan;173(2):487–494. doi: 10.1128/jb.173.2.487-494.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Leon O., Panos C. Adherence of Streptococcus agalactiae to synchronously growing human cell monolayers without lipoteichoic acid involvement. Infect Immun. 1988 Feb;56(2):505–512. doi: 10.1128/iai.56.2.505-512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect Immun. 1984 Feb;43(2):523–530. doi: 10.1128/iai.43.2.523-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J. Physiology and ecology of the vagina. Scand J Infect Dis Suppl. 1983;40:31–35. [PubMed] [Google Scholar]
- Raff H. V., Siscoe P. J., Wolff E. A., Maloney G., Shuford W. Human monoclonal antibodies to group B streptococcus. Reactivity and in vivo protection against multiple serotypes. J Exp Med. 1988 Sep 1;168(3):905–917. doi: 10.1084/jem.168.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Raff H. V., Jackson J. C., Chi E. Y., Bielitzki J. T., Hillier S. L. Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J Infect Dis. 1991 Aug;164(2):320–330. doi: 10.1093/infdis/164.2.320. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Smith S., Hulse M., Chi E. Y., van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992 Dec;60(12):5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti G., Tomasello F., Chiofalo M. S., Orefici G., Mastroeni P. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun. 1987 Dec;55(12):3057–3064. doi: 10.1128/iai.55.12.3057-3064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibawan I. T., Lämmler C., Pasaribu F. H. Role of hydrophobic surface proteins in mediating adherence of group B streptococci to epithelial cells. J Gen Microbiol. 1992 Jun;138(6):1237–1242. doi: 10.1099/00221287-138-6-1237. [DOI] [PubMed] [Google Scholar]
- Wibawan I. W., Lämmler C. Influence of capsular neuraminic acid on properties of streptococci of serological group B. J Gen Microbiol. 1991 Dec;137(12):2721–2725. doi: 10.1099/00221287-137-12-2721. [DOI] [PubMed] [Google Scholar]
- Wrong O., Metcalfegibson A. The electrolyte content faeces. Proc R Soc Med. 1965 Dec;58(12):1007–1009. doi: 10.1177/003591576505801203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Endo S., Yokota T., Echeverria P. Characteristics of adherence of enteroaggregative Escherichia coli to human and animal mucosa. Infect Immun. 1991 Oct;59(10):3722–3739. doi: 10.1128/iai.59.10.3722-3739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kamano T., Uchimura M., Iwanaga M., Yokota T. Vibrio cholerae O1 adherence to villi and lymphoid follicle epithelium: in vitro model using formalin-treated human small intestine and correlation between adherence and cell-associated hemagglutinin levels. Infect Immun. 1988 Dec;56(12):3241–3250. doi: 10.1128/iai.56.12.3241-3250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Adherence targets of Vibrio parahaemolyticus in human small intestines. Infect Immun. 1989 Aug;57(8):2410–2419. doi: 10.1128/iai.57.8.2410-2419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawaneh S. M., Ayoub E. M., Baer H., Cruz A. C., Spellacy W. N. Factors influencing adherence of group B streptococci to human vaginal epithelial cells. Infect Immun. 1979 Nov;26(2):441–447. doi: 10.1128/iai.26.2.441-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



