Abstract
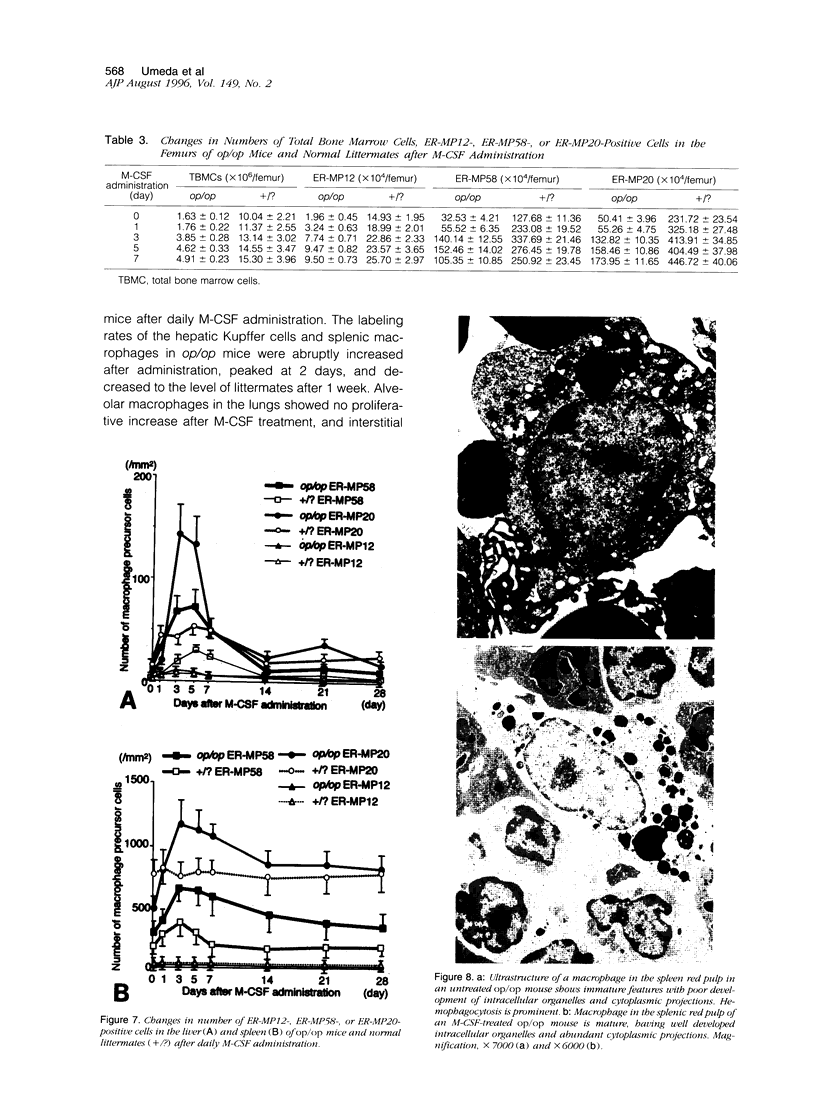
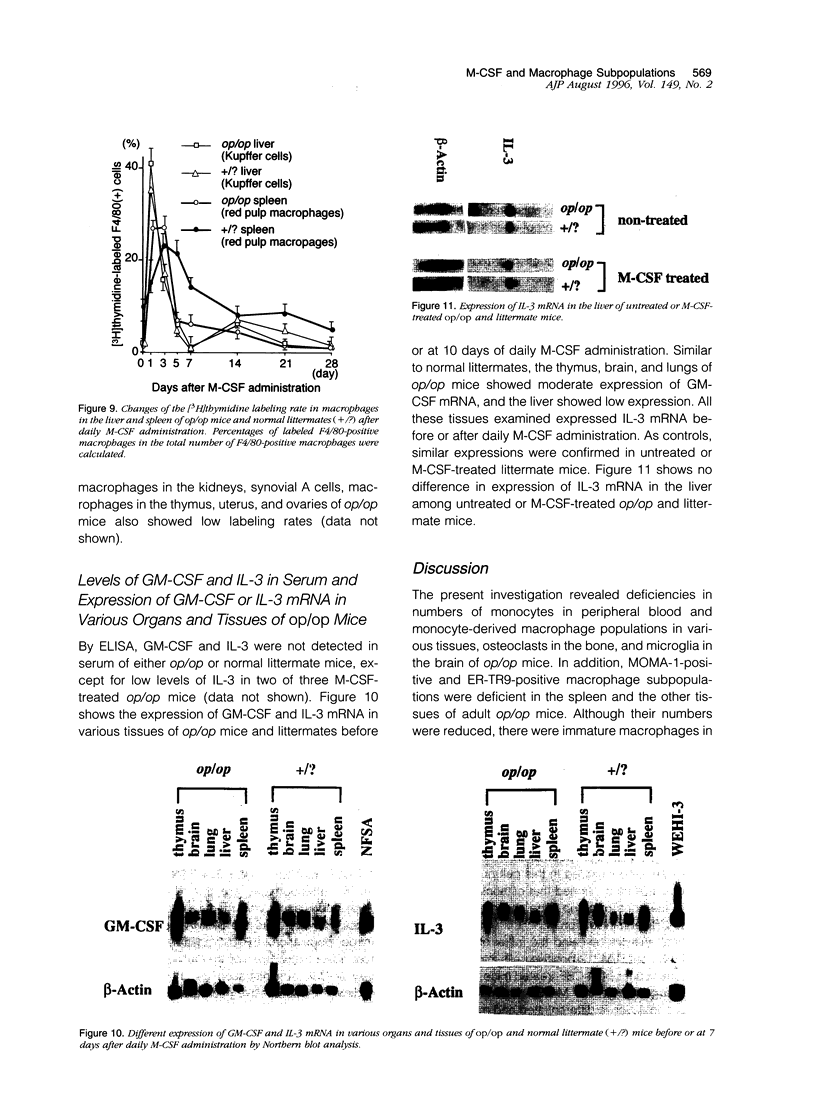
The development of macrophage populations in osteopetrosis (op) mutant mice defective in production of functional macrophage colony-stimulating factor (M-CSF) and the response of these cell populations to exogenous M-CSF were used to classify macrophages into four groups: 1) monocytes, monocyte-derived macrophages, and osteoclasts, 2) MOMA-1-positive macrophages, 3) ER-TR9-positive macrophages, and 4) immature tissue macrophages. Monocytes, monocyte-derived macrophages, osteoclasts in bone, microglia in brain, synovial A cells, and MOMA-1- or ER-TR9-positive macrophages were deficient in op/op mice. The former three populations expanded to normal levels in op/op mice after daily M-CSF administration, indicating that they are developed and differentiated due to the effect of M-CSF supplied humorally. In contrast, the other cells did not respond or very slightly responded to M-CSF, and their development seems due to either M-CSF produced in situ or expression of receptor for M-CSF. Macrophages present in tissues of the mutant mice were immature and appear to be regulated by either granulocyte/macrophage colony-stimulating factor and/or interleukin-3 produced in situ or receptor expression. Northern blot analysis revealed different expressions of GM-CSF and IL-3 mRNA in various tissues of the op/op mice. However, granulocyte/macrophage colony-stimulating factor and interleukin-3 in serum were not detected by enzyme-linked immunosorbent assay. The immature macrophages differentiated and matured into resident macrophages after M-CSF administration, and some of these cells proliferated in response to M-CSF.
Full text
PDF


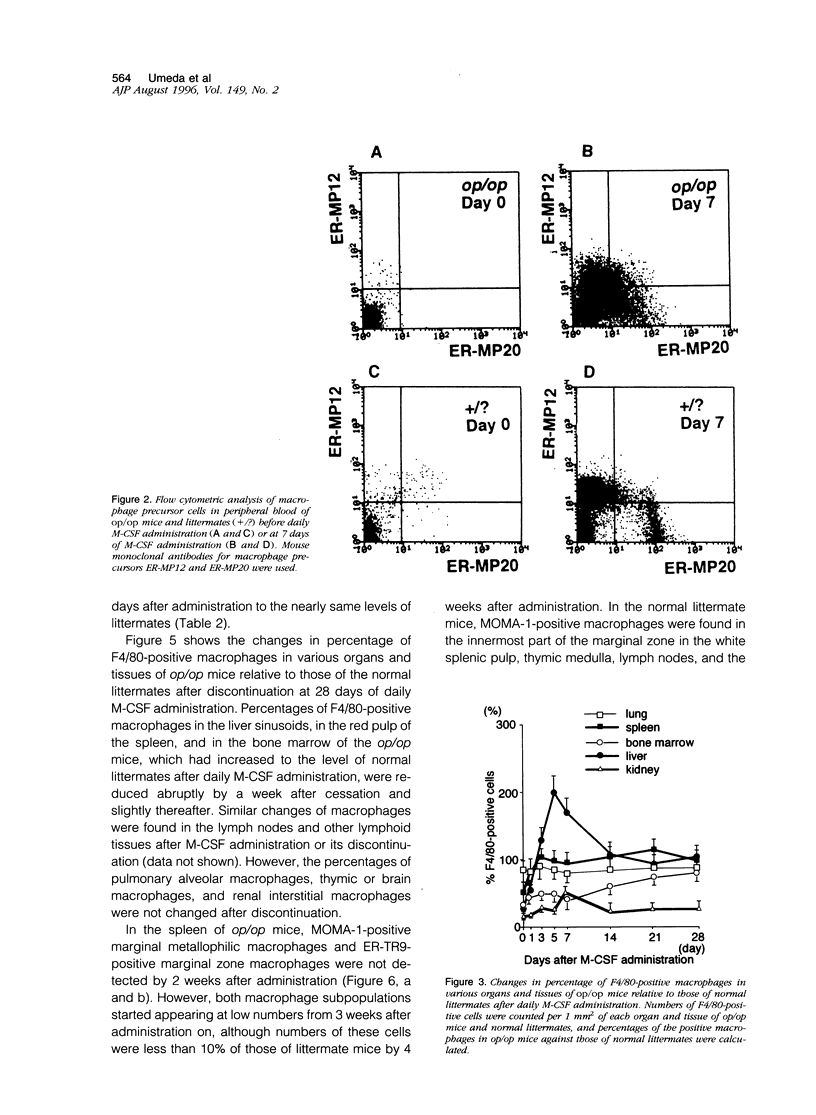
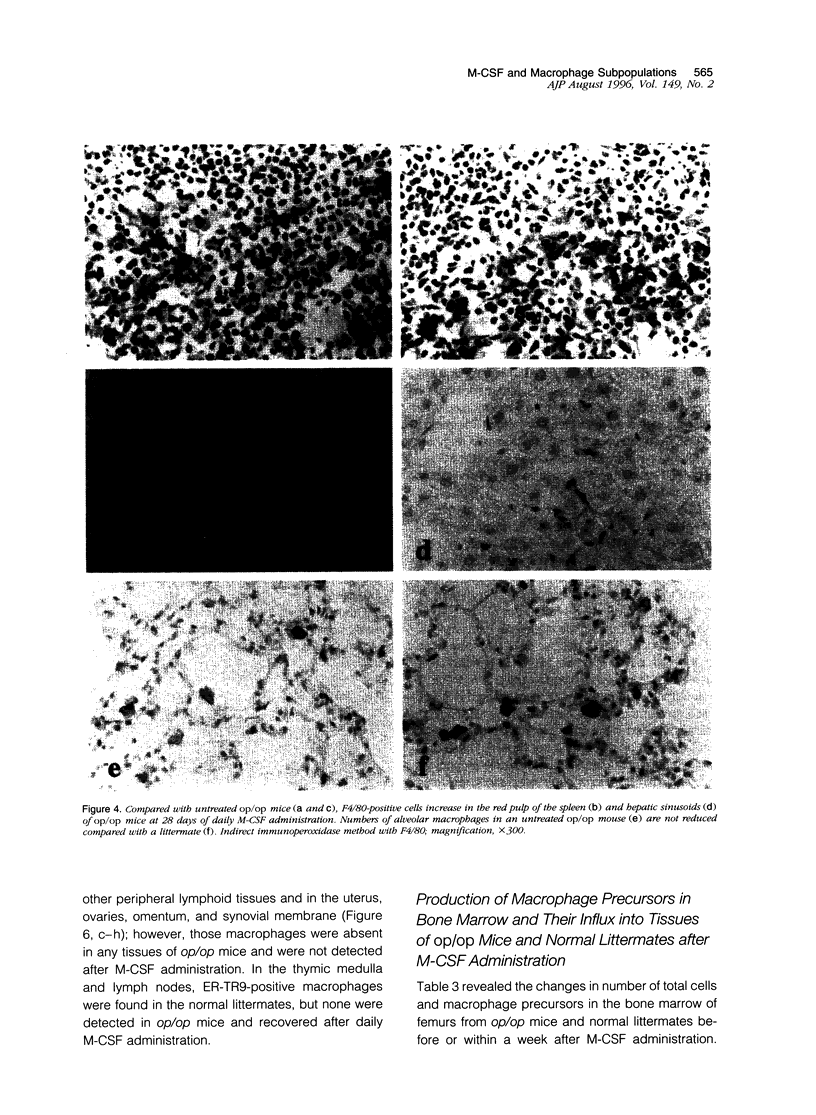
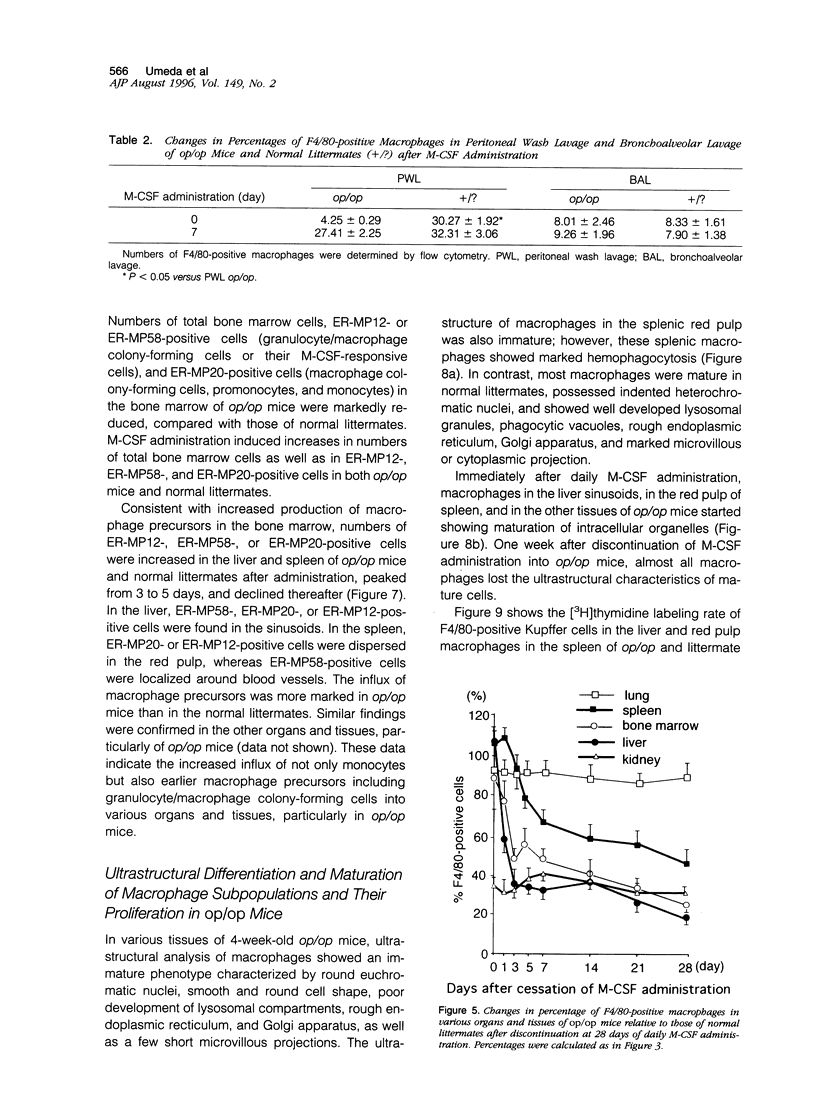
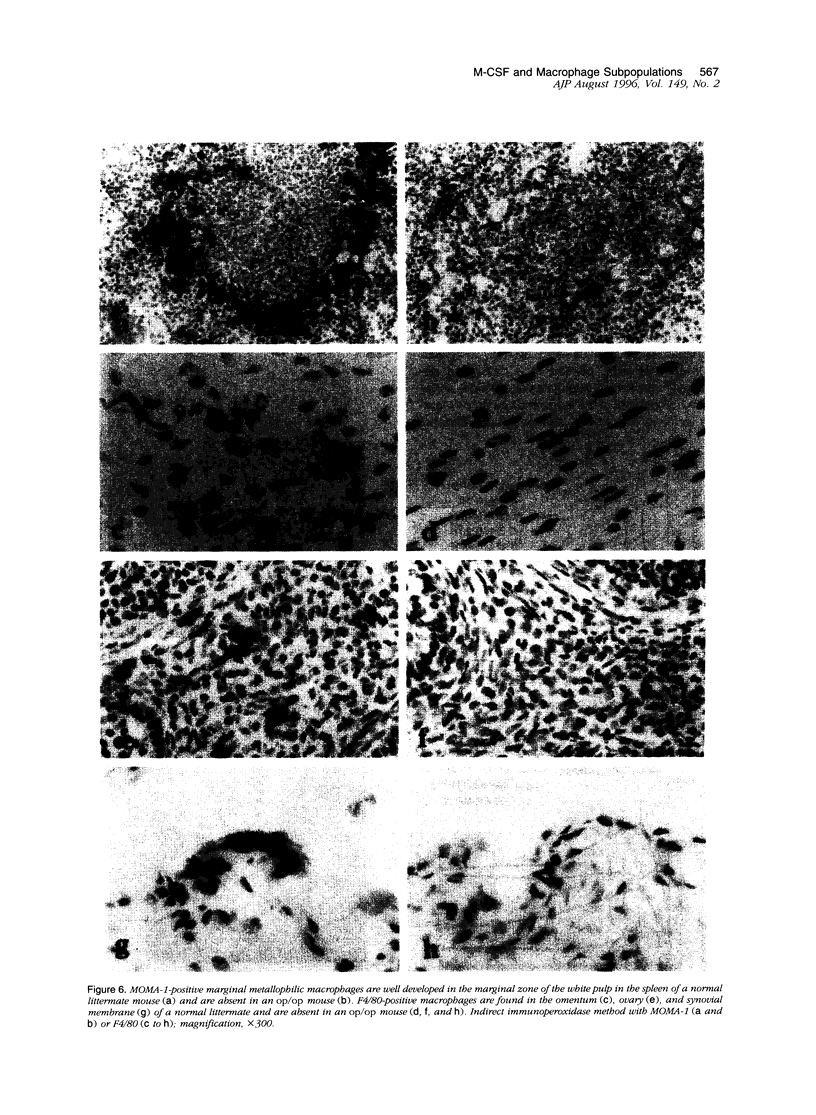





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa K. S., Kamoshita K., Tokunaga T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J Immunol. 1988 Nov 15;141(10):3383–3390. [PubMed] [Google Scholar]
- Bagley C. J., Woodcock J. M., Hercus T. R., Shannon M. F., Lopez A. F. Interaction of GM-CSF and IL-3 with the common beta-chain of their receptors. J Leukoc Biol. 1995 May;57(5):739–746. doi: 10.1002/jlb.57.5.739. [DOI] [PubMed] [Google Scholar]
- Cammer W., Sacchi R., Sapirstein V. Immunocytochemical localization of carbonic anhydrase in the spinal cords of normal and mutant (shiverer) adult mice with comparisons among fixation methods. J Histochem Cytochem. 1985 Jan;33(1):45–54. doi: 10.1177/33.1.3917467. [DOI] [PubMed] [Google Scholar]
- Cecchini M. G., Dominguez M. G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J. W., Stanley E. R. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994 Jun;120(6):1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Loutit J. F. A functional assessment of macrophages from osteopetrotic mice. J Pathol. 1979 Oct;129(2):57–63. doi: 10.1002/path.1711290203. [DOI] [PubMed] [Google Scholar]
- Claassen E., Ott A., Boersma W. J., Deen C., Schellekens M. M., Dijkstra C. D., Kors N., Van Rooijen N. Marginal zone of the murine spleen in autotransplants: functional and histological observations in the response against a thymus-independent type 2 antigen. Clin Exp Immunol. 1989 Sep;77(3):445–451. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Van Vliet E., Döpp E. A., van der Lelij A. A., Kraal G. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 1985 May;55(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Elomaa O., Kangas M., Sahlberg C., Tuukkanen J., Sormunen R., Liakka A., Thesleff I., Kraal G., Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995 Feb 24;80(4):603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Yang Y. C., Clark S. C., Long M. W. Human recombinant granulocyte-macrophage colony stimulating factor and interleukin 3 have overlapping but distinct hematopoietic activities. J Clin Invest. 1988 Oct;82(4):1282–1287. doi: 10.1172/JCI113727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Hofstetter W., Elford P. R., Stutzer A., Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990 Jul;5(7):781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- Honda Y., Takahashi K., Naito M., Fujiyama S. The role of macrophage colony-stimulating factor in the differentiation and proliferation of Kupffer cells in the liver of protein-deprived mice. Lab Invest. 1995 Jun;72(6):696–706. [PubMed] [Google Scholar]
- Hume D. A., Loutit J. F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984 Mar;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. H. Tolerogenic or immunogenic activity of hapten-conjugated polysaccharides correlated with cellular localization. Eur J Immunol. 1981 Mar;11(3):212–220. doi: 10.1002/eji.1830110310. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Abe M., Niida S., Hakeda Y., Kawashima H. Transient recruitment of osteoclasts and expression of their function in osteopetrotic (op/op) mice by a single injection of macrophage colony-stimulating factor. J Bone Miner Res. 1993 Jan;8(1):45–50. doi: 10.1002/jbmr.5650080107. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Janse M., Claassen E. Marginal metallophilic macrophages in the mouse spleen: effects of neonatal injections of MOMA-1 antibody on the humoral immune response. Immunol Lett. 1988 Feb;17(2):139–144. doi: 10.1016/0165-2478(88)90082-x. [DOI] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Ter Hart H., Meelhuizen C., Venneker G., Claassen E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur J Immunol. 1989 Apr;19(4):675–680. doi: 10.1002/eji.1830190416. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Hapel A. J., Ihle J. N. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. 1982 Jun;128(6):2393–2398. [PubMed] [Google Scholar]
- Leenen P. J., Melis M., Slieker W. A., Van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990 Jan;20(1):27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., de Bruijn M. F., Voerman J. S., Campbell P. A., van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994 Sep 14;174(1-2):5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Malorny U., Michels E., Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243(2):421–428. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Lane P. W. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976 Jan-Feb;67(1):11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr Morphological evidence of reduced bone resorption in osteopetrotic (op) mice. Am J Anat. 1982 Feb;163(2):157–167. doi: 10.1002/aja.1001630205. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Seifert M. F., McGuire J. L. Congenitally osteopetrotic (oplop) mice are not cured by transplants of spleen or bone marrow cells from normal littermates. Metab Bone Dis Relat Res. 1984;5(4):183–186. doi: 10.1016/0221-8747(84)90027-4. [DOI] [PubMed] [Google Scholar]
- McCormack J. M., Leenen P. J., Walker W. S. Macrophage progenitors from mouse bone marrow and spleen differ in their expression of the Ly-6C differentiation antigen. J Immunol. 1993 Dec 1;151(11):6389–6398. [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Morioka Y., Naito M., Sato T., Takahashi K. Immunophenotypic and ultrastructural heterogeneity of macrophage differentiation in bone marrow and fetal hematopoiesis of mouse in vitro and in vivo. J Leukoc Biol. 1994 May;55(5):642–651. doi: 10.1002/jlb.55.5.642. [DOI] [PubMed] [Google Scholar]
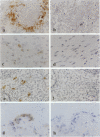
- Naito M., Hayashi S., Yoshida H., Nishikawa S., Shultz L. D., Takahashi K. Abnormal differentiation of tissue macrophage populations in 'osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991 Sep;139(3):657–667. [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Akagawa K. S., Fukayama M., Hayashi Y., Kadokura M., Tokunaga T. Granulocyte-macrophage colony-stimulating factor promotes the proliferation of human alveolar macrophages in vitro. J Immunol. 1991 Aug 15;147(4):1266–1272. [PubMed] [Google Scholar]
- Nishinakamura R., Nakayama N., Hirabayashi Y., Inoue T., Aud D., McNeil T., Azuma S., Yoshida S., Toyoda Y., Arai K. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995 Mar;2(3):211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Oghiso Y., Yamada Y., Ando K., Ishihara H., Shibata Y. Differential induction of prostaglandin E2-dependent and -independent immune suppressor cells by tumor-derived GM-CSF and M-CSF. J Leukoc Biol. 1993 Jan;53(1):86–92. doi: 10.1002/jlb.53.1.86. [DOI] [PubMed] [Google Scholar]
- SNOOK T. STUDIES ON THE PERIFOLLICULAR REGION OF THE RAT'S SPLEEN. Anat Rec. 1964 Feb;148:149–159. doi: 10.1002/ar.1091480205. [DOI] [PubMed] [Google Scholar]
- Sonoda Y., Yang Y. C., Wong G. G., Clark S. C., Ogawa M. Analysis in serum-free culture of the targets of recombinant human hemopoietic growth factors: interleukin 3 and granulocyte/macrophage-colony-stimulating factor are specific for early developmental stages. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4360–4364. doi: 10.1073/pnas.85.12.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Stanley E., Lieschke G. J., Grail D., Metcalf D., Hodgson G., Gall J. A., Maher D. W., Cebon J., Sinickas V., Dunn A. R. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Naito M., Umeda S., Shultz L. D. The role of macrophage colony-stimulating factor in hepatic glucan-induced granuloma formation in the osteopetrosis mutant mouse defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1994 Jun;144(6):1381–1392. [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Umeda S., Shultz L. D., Hayashi S., Nishikawa S. Effects of macrophage colony-stimulating factor (M-CSF) on the development, differentiation, and maturation of marginal metallophilic macrophages and marginal zone macrophages in the spleen of osteopetrosis (op) mutant mice lacking functional M-CSF activity. J Leukoc Biol. 1994 May;55(5):581–588. doi: 10.1002/jlb.55.5.581. [DOI] [PubMed] [Google Scholar]
- Tominaga A., Mita S., Kikuchi Y., Hitoshi Y., Takatsu K., Nishikawa S., Ogawa M. Establishment of IL-5-dependent early B cell lines by long-term bone marrow cultures. Growth Factors. 1989;1(2):135–146. doi: 10.3109/08977198909029123. [DOI] [PubMed] [Google Scholar]
- Umeda S., Takahashi K., Naito M., Shultz L. D., Takagi K. Neonatal changes of osteoclasts in osteopetrosis (op/op) mice defective in production of functional macrophage colony-stimulating factor (M-CSF) protein and effects of M-CSF on osteoclast development and differentiation. J Submicrosc Cytol Pathol. 1996 Jan;28(1):13–26. [PubMed] [Google Scholar]
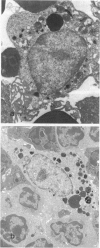
- Usuda H., Naito M., Umeda S., Takahashi K., Shultz L. D. Ultrastructure of macrophages and dendritic cells in osteopetrosis (op) mutant mice lacking macrophage colony-stimulating factor (M-CSF/CSF-1) activity. J Submicrosc Cytol Pathol. 1994 Jan;26(1):111–119. [PubMed] [Google Scholar]
- Wijffels J. F., de Rover Z., Kraal G., Beelen R. H. Macrophage phenotype regulation by colony-stimulating factors at bone marrow level. J Leukoc Biol. 1993 Mar;53(3):249–255. doi: 10.1002/jlb.53.3.249. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Ratajczak M. Z., Ptasznik A., Sell K. W., Ahmed-Ansari A., Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992 Sep;20(8):1004–1010. [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Urbanowska E., Aukerman S. L., Pollard J. W., Stanley E. R., Ralph P., Ansari A. A., Sell K. W., Szperl M. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp Hematol. 1991 Nov;19(10):1049–1054. [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Urbanowska E., Szperl M. Granulocyte-macrophage colony-stimulating factor corrects macrophage deficiencies, but not osteopetrosis, in the colony-stimulating factor-1-deficient op/op mouse. Endocrinology. 1994 Apr;134(4):1932–1935. doi: 10.1210/endo.134.4.8137761. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. F., Slieker W. A., van der Loo J. C., Voerman J. S., van Ewijk W., Leenen P. J. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994 Oct;24(10):2279–2284. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989 Feb;45(2):97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- van der Loo J. C., Slieker W. A., Kieboom D., Ploemacher R. E. Identification of hematopoietic stem cell subsets on the basis of their primitiveness using antibody ER-MP12. Blood. 1995 Feb 15;85(4):952–962. [PubMed] [Google Scholar]