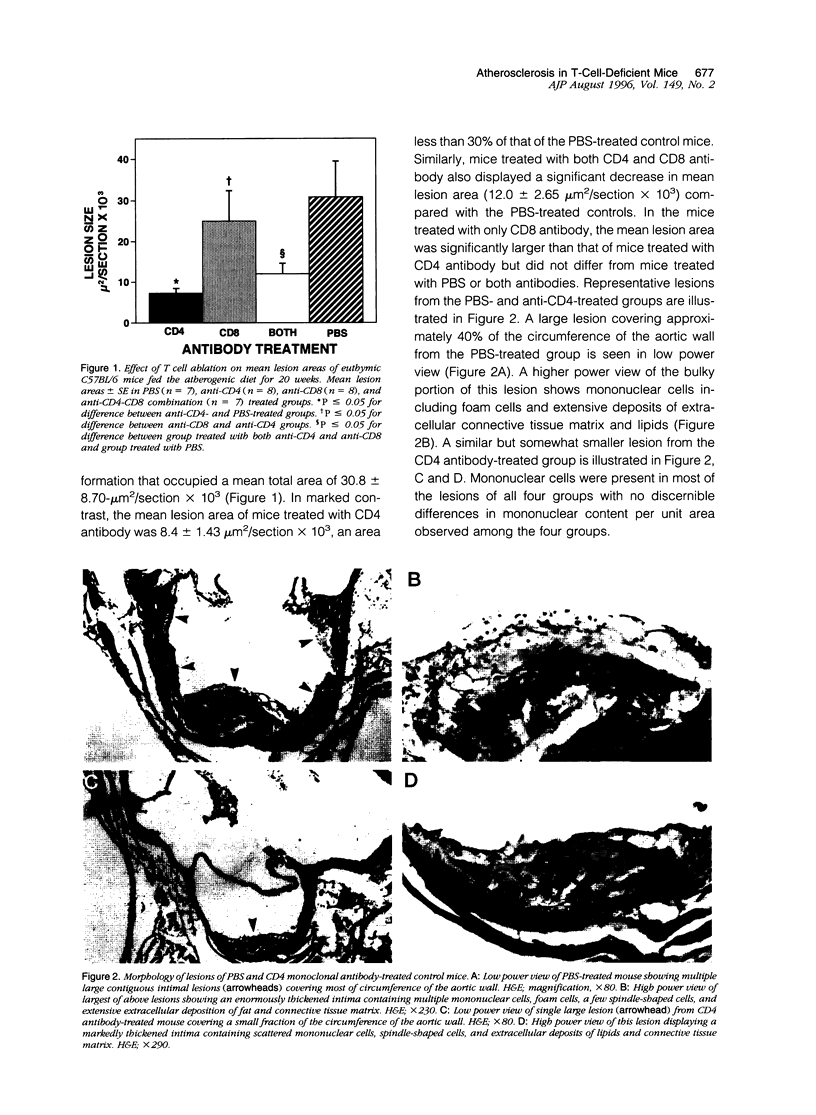
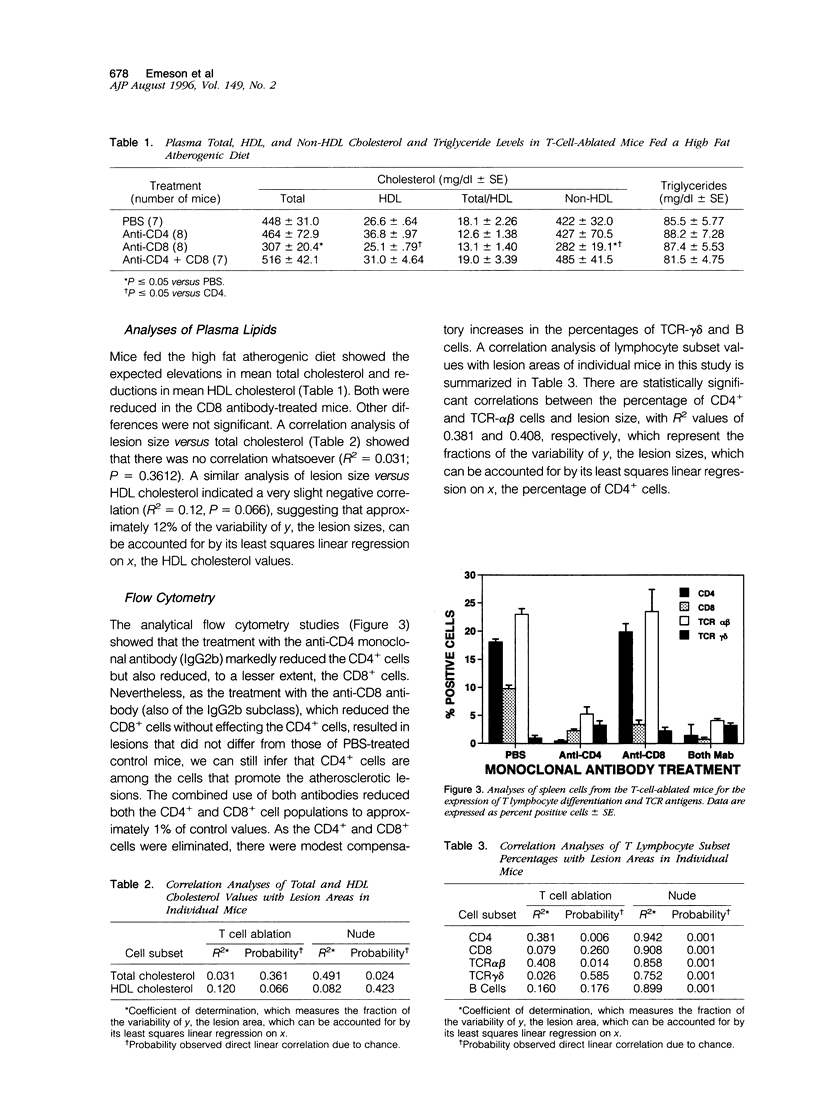
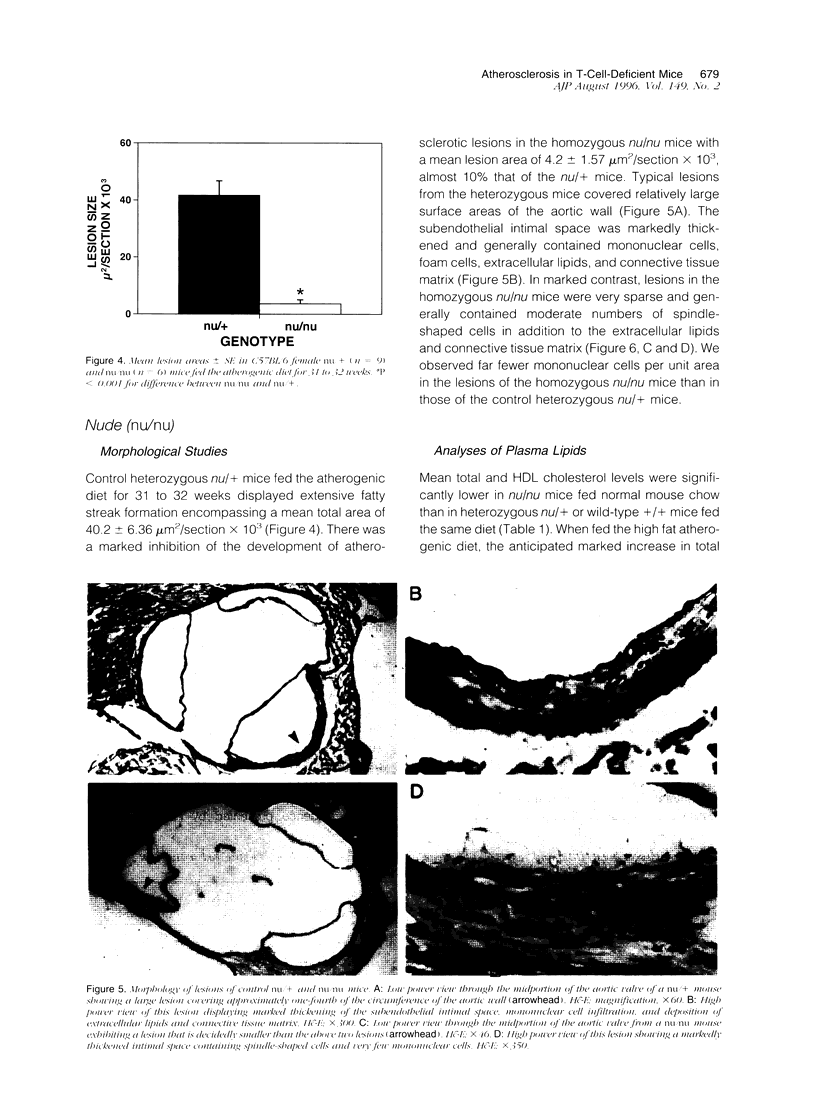
Abstract
T lymphocytes and monocyte/macrophages are prominent components of atherosclerotic lesions, and many of these cells are activated and secreting cytokines. To determine the role of these cells in the pathogenesis of atherosclerosis, we studied its development in T-cell-deficient mice fed a high fat atherogenic diet. Depleting euthymic mice of their CD4+ lymphocytes by 20 weekly injections of CD4 monoclonal antibodies reduced the mean area of their aortic lesions by approximately 70%. Similarly, the mean lesion area of T-cell-deficient nude (nu/nu) mice was 10% of the size of that of their heterozygote (nu/+) litter mates. Flow cytometric studies of splenic T cells and analyses of serum total and HDL cholesterol of these mice indicated that the differences in mean lesion areas among the experimental groups were most closely correlated with differences in splenic T cells content. These studies suggest that in these two models T lymphocytes contribute to the pathogenesis of early atherosclerotic lesions and that a further understanding of this phenomenon may provide future approaches toward the prevention and treatment of the disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballantyne C. M., Podet E. J., Patsch W. P., Harati Y., Appel V., Gotto A. M., Jr, Young J. B. Effects of cyclosporine therapy on plasma lipoprotein levels. JAMA. 1989 Jul 7;262(1):53–56. [PubMed] [Google Scholar]
- Bunchman T. E., Brookshire C. A. Cyclosporine-induced synthesis of endothelin by cultured human endothelial cells. J Clin Invest. 1991 Jul;88(1):310–314. doi: 10.1172/JCI115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcamuggi G., Lanzio M., Babini G., Martini S., Anfossi G., Emanuelli G. Sodium taurocholate affects prostacyclin constitutive production by cultured human vascular endothelial cells. J Lab Clin Med. 1990 Jun;115(6):756–760. [PubMed] [Google Scholar]
- Callow M. J., Verstuyft J., Tangirala R., Palinski W., Rubin E. M. Atherogenesis in transgenic mice with human apolipoprotein B and lipoprotein (a). J Clin Invest. 1995 Sep;96(3):1639–1646. doi: 10.1172/JCI118203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartsch P. C., Bauriedel G., Schinko I., Weiss H. D., Höfling B., Betz E. Cell constitution and characteristics of human atherosclerotic plaques selectively removed by percutaneous atherectomy. Atherosclerosis. 1989 Dec;80(2):149–157. doi: 10.1016/0021-9150(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Drew A. F., Tipping P. G. Cyclosporine treatment reduces early atherosclerosis in the cholesterol-fed rabbit. Atherosclerosis. 1995 Aug;116(2):181–189. doi: 10.1016/0021-9150(95)05539-9. [DOI] [PubMed] [Google Scholar]
- Dueland S., Drisko J., Graf L., Machleder D., Lusis A. J., Davis R. A. Effect of dietary cholesterol and taurocholate on cholesterol 7 alpha-hydroxylase and hepatic LDL receptors in inbred mice. J Lipid Res. 1993 Jun;34(6):923–931. [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Reidy M. A., Ross R. Balloon catheter de-endothelialization of the nude rat carotid. Response to injury in the absence of functional T lymphocytes. Am J Pathol. 1991 Apr;138(4):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- Fyfe A. I., Qiao J. H., Lusis A. J. Immune-deficient mice develop typical atherosclerotic fatty streaks when fed an atherogenic diet. J Clin Invest. 1994 Dec;94(6):2516–2520. doi: 10.1172/JCI117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. M., Mills C. O., Elias E., Neuberger J. M. The effect of bile salts on human vascular endothelial cells. Biochim Biophys Acta. 1991 Jan 10;1091(1):41–45. doi: 10.1016/0167-4889(91)90219-n. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Grainger D. J., Kemp P. R., Metcalfe J. C., Liu A. C., Lawn R. M., Williams N. R., Grace A. A., Schofield P. M., Chauhan A. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med. 1995 Jan;1(1):74–79. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- Grainger D. J., Witchell C. M., Metcalfe J. C. Tamoxifen elevates transforming growth factor-beta and suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med. 1995 Oct;1(10):1067–1073. doi: 10.1038/nm1095-1067. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Glimcher L. H. Immune responses in MHC class II-deficient mice. Annu Rev Immunol. 1995;13:417–435. doi: 10.1146/annurev.iy.13.040195.002221. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Adams D. H., Wyner L. R., Sayegh M. H., Karnovsky M. J. CD4+ mononuclear cells induce cytokine expression, vascular smooth muscle cell proliferation, and arterial occlusion after endothelial injury. Am J Pathol. 1994 Nov;145(5):1008–1014. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Holm S., Fotev Z., Hedrich H. J., Fingerle J. T lymphocytes inhibit the vascular response to injury. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10530–10534. doi: 10.1073/pnas.88.23.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Asano Y., Matsuoka S., Furutani-Seiki M., Aizawa S., Nishimura H., Shirai T., Tada T. Distinction of mouse CD8+ suppressor effector T cell clones from cytotoxic T cell clones by cytokine production and CD45 isoforms. J Immunol. 1993 Mar 15;150(6):2121–2128. [PubMed] [Google Scholar]
- Ishibashi S., Goldstein J. L., Brown M. S., Herz J., Burns D. K. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994 May;93(5):1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Lawetzky A., Hünig T. Analysis of CD3 and antigen receptor expression on T cell subpopulations of aged athymic mice. Eur J Immunol. 1988 Mar;18(3):409–416. doi: 10.1002/eji.1830180314. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Wade D. P., Hammer R. E., Chiesa G., Verstuyft J. G., Rubin E. M. Atherogenesis in transgenic mice expressing human apolipoprotein(a) Nature. 1992 Dec 17;360(6405):670–672. doi: 10.1038/360670a0. [DOI] [PubMed] [Google Scholar]
- Libby P., Galis Z. S. Cytokines regulate genes involved in atherogenesis. Ann N Y Acad Sci. 1995 Jan 17;748:158–170. doi: 10.1111/j.1749-6632.1994.tb17315.x. [DOI] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Liblau R. S., Singer S. M., McDevitt H. O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995 Jan;16(1):34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- Lopes-Virella M. F., Stone P., Ellis S., Colwell J. A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977 May;23(5):882–884. [PubMed] [Google Scholar]
- Lustig S., Stern N., Golub M. S., Eggena P., Barrett J., Lee D. B. Experimental cyclosporin hypertension: characterization of the rat model. Transplant Proc. 1989 Feb;21(1 Pt 1):950–951. [PubMed] [Google Scholar]
- MacDonald H. R., Blanc C., Lees R. K., Sordat B. Abnormal distribution of T cell subsets in athymic mice. J Immunol. 1986 Jun 15;136(12):4337–4339. [PubMed] [Google Scholar]
- Marotti K. R., Castle C. K., Boyle T. P., Lin A. H., Murray R. W., Melchior G. W. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature. 1993 Jul 1;364(6432):73–75. doi: 10.1038/364073a0. [DOI] [PubMed] [Google Scholar]
- Mason D., Fowell D. T-cell subsets in autoimmunity. Curr Opin Immunol. 1992 Dec;4(6):728–732. doi: 10.1016/0952-7915(92)90053-h. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. T-cell subsets in autoimmunity. Curr Opin Immunol. 1993 Dec;5(6):880–886. doi: 10.1016/0952-7915(93)90100-7. [DOI] [PubMed] [Google Scholar]
- Paigen B., Morrow A., Holmes P. A., Mitchell D., Williams R. A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987 Dec;68(3):231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- Palinski W., Miller E., Witztum J. L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Purcell-Huynh D. A., Farese R. V., Jr, Johnson D. F., Flynn L. M., Pierotti V., Newland D. L., Linton M. F., Sanan D. A., Young S. G. Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J Clin Invest. 1995 May;95(5):2246–2257. doi: 10.1172/JCI117915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw A. L., Parums D. V. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990 Dec;17(6):543–552. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Reddick R. L., Zhang S. H., Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994 Jan;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Boulay J. L., Finkelman F., Barbier S., Ben-Sasson S. Z., Le Gros G., Paul W. E. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992 Mar 15;148(6):1652–1656. [PubMed] [Google Scholar]
- Stemme S., Faber B., Holm J., Wiklund O., Witztum J. L., Hansson G. K. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Schett G., Amberger A., Kleindienst R., Xu Q. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995 Jan;16(1):27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- Xu Q. B., Oberhuber G., Gruschwitz M., Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990 Sep;56(3):344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Xu Q., Kleindienst R., Waitz W., Dietrich H., Wick G. Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J Clin Invest. 1993 Jun;91(6):2693–2702. doi: 10.1172/JCI116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zoja C., Furci L., Ghilardi F., Zilio P., Benigni A., Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986 Oct;55(4):455–462. [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Bentz van de Berg D., van der Loos C. M., Becker A. E. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989 Aug;61(2):166–170. [PubMed] [Google Scholar]