Abstract
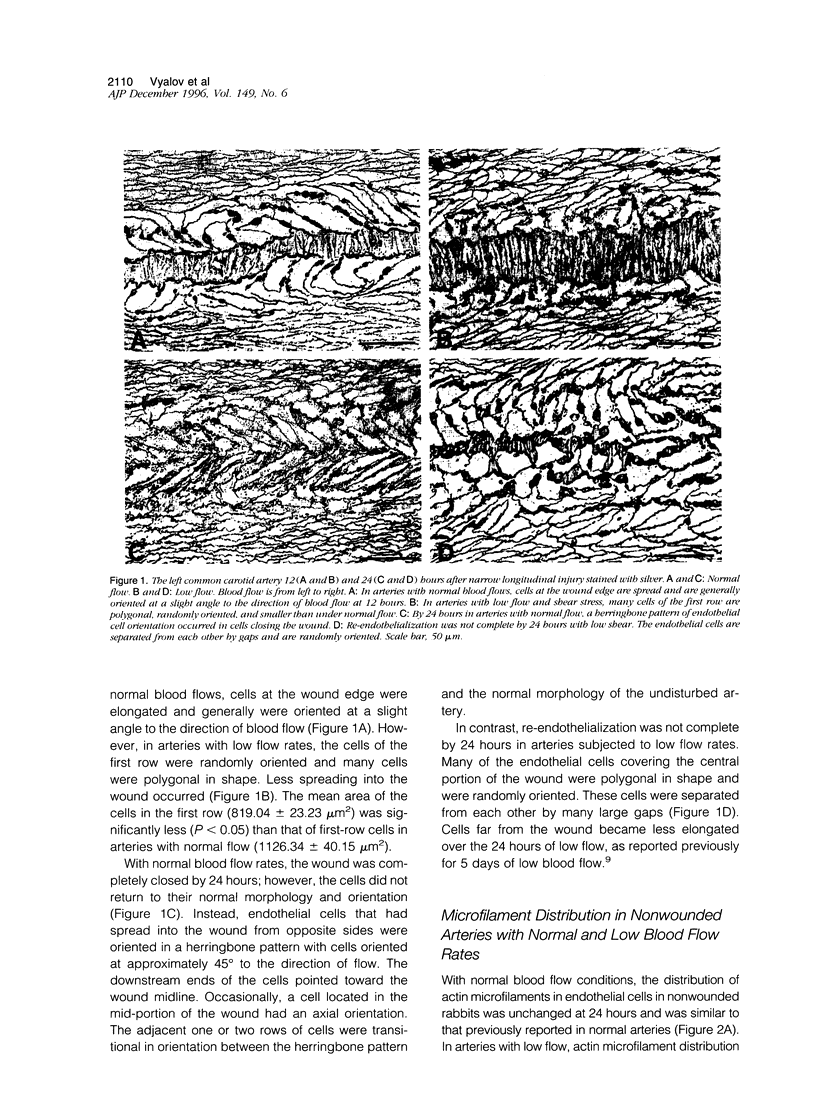
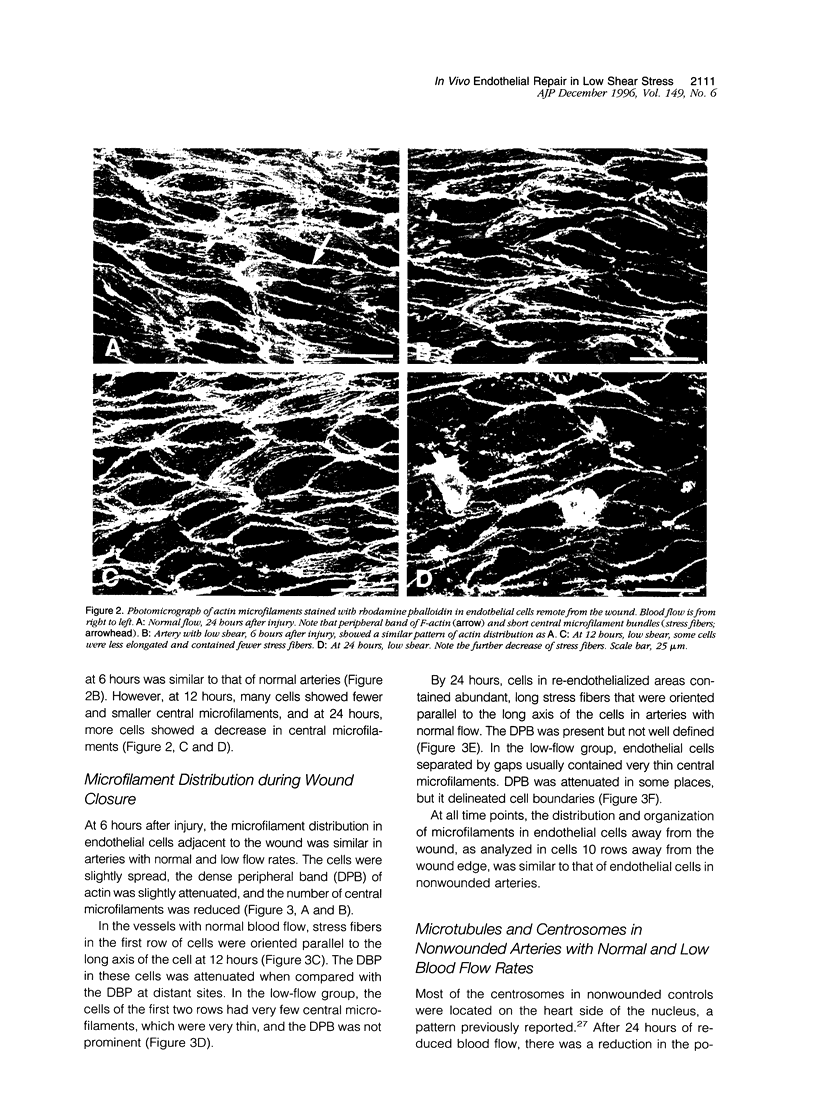
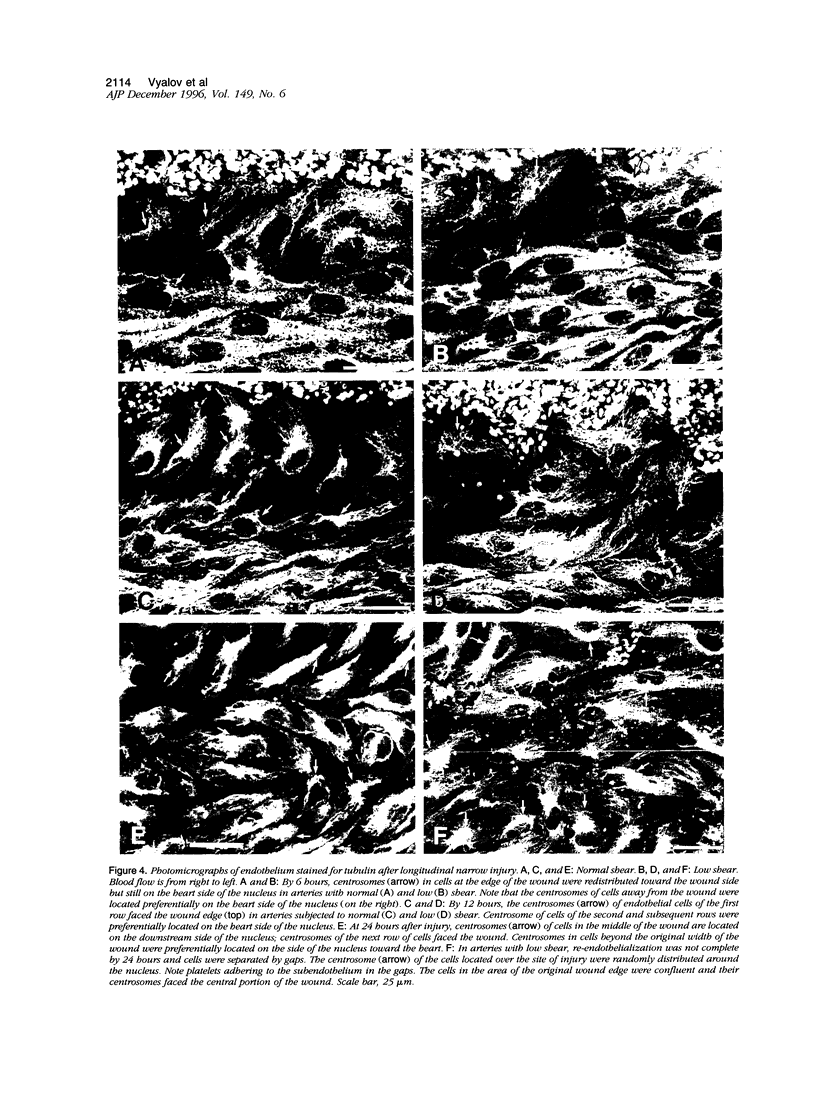
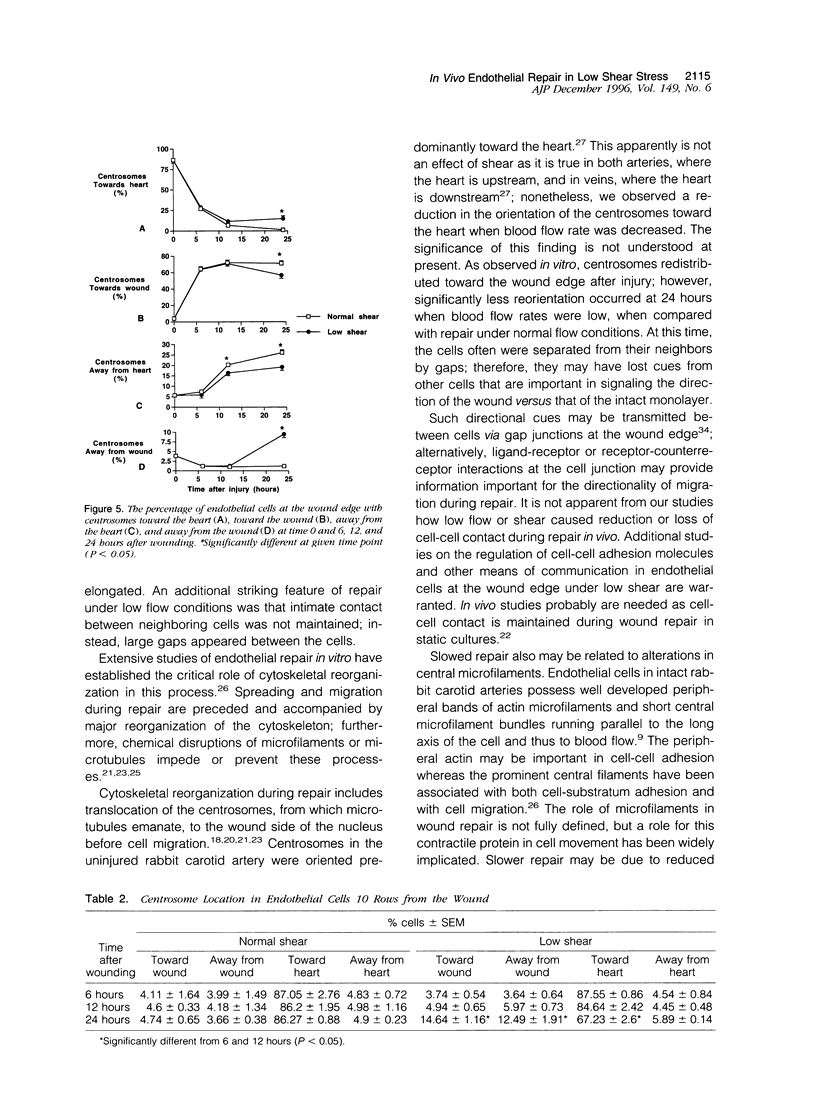
Both local hemodynamics and endothelial injury have been implicated in vascular disorders including bypass graft failure and atherogenesis, but little is known about the effect of local blood flow conditions on repair of endothelial injury. We decreased blood flow rates and shear stresses in common carotid arteries of rabbits by ligating the ipsilateral external carotid artery. After 24 hours, endothelial cells were less elongated, contained fewer central microfilament bundles, and showed less polarity of the centrosome toward the heart than endothelial cells in unmanipulated carotid arteries. To examine wound repair, we made narrow longitudinal intimal wounds at the time of flow reduction using a nylon monofilament device. In arteries with normal blood flows, endothelial cells at the edge of the wound initially spread and elongated in the direction of the wound. The dense peripheral band of actin was attenuated and central microfilaments became more prominent. Endothelial cells remained in close contact with their neighbors in the monolayer. The centrosome of cells adjacent to the wound was redistributed toward the wound side of the nucleus at 6 and 12 hours. Complete closure occurred by 24 hours, at which time the elongated endothelial cells covering the wound were organized in a herringbone pattern with their downstream ends at the center of the wound. With decreased flow and shear stress, the cells at the wound edge spread less than those in normal vessels at 12 hours after wounding and were randomly oriented and polygonal in shape. Also, re-endothelialization proceeded more slowly and there was a marked reduction of central microfilaments in cells at the wound edge. At 24 hours, the wounds were still open, the endothelial cells covering the central portion of the wound did not maintain intimate contact with their neighbors, and orientation of the centrosome toward the wound was reduced. We hypothesize that loss of cell-cell contact during repair at low flow rates and low shear stress disrupts intercellular communication and results in disruption of cytoskeletal reorganization during repair, thereby slowing the repair process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell L., Madri J. A. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res. 1989 Oct;65(4):1057–1065. doi: 10.1161/01.res.65.4.1057. [DOI] [PubMed] [Google Scholar]
- Davies P. F. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995 Jul;75(3):519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenson D. S., Gotlieb A. I. Basic fibroblast growth factor is a signal for the initiation of centrosome redistribution to the front of migrating endothelial cells at the edge of an in vitro wound. Arterioscler Thromb Vasc Biol. 1995 Apr;15(4):515–521. doi: 10.1161/01.atv.15.4.515. [DOI] [PubMed] [Google Scholar]
- Ettenson D. S., Gotlieb A. I. Centrosomes, microtubules, and microfilaments in the reendothelialization and remodeling of double-sided in vitro wounds. Lab Invest. 1992 Jun;66(6):722–733. [PubMed] [Google Scholar]
- Ettenson D. S., Gotlieb A. I. In vitro large-wound re-endothelialization. Inhibition of centrosome redistribution by transient inhibition of transcription after wounding prevents rapid repair. Arterioscler Thromb. 1993 Sep;13(9):1270–1281. doi: 10.1161/01.atv.13.9.1270. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Regulation of production of a platelet-derived growth factor-like protein by cultured bovine aortic endothelial cells. J Cell Physiol. 1984 Nov;121(2):298–308. doi: 10.1002/jcp.1041210206. [DOI] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W., Wong M. K., Lacey C. In vitro reendothelialization. Microfilament bundle reorganization in migrating porcine endothelial cells. Arteriosclerosis. 1984 Mar-Apr;4(2):91–96. doi: 10.1161/01.atv.4.2.91. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., Subrahmanyan L., Kalnins V. I. Microtubule-organizing centers and cell migration: effect of inhibition of migration and microtubule disruption in endothelial cells. J Cell Biol. 1983 May;96(5):1266–1272. doi: 10.1083/jcb.96.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A. I., Langille B. L., Wong M. K., Kim D. W. Structure and function of the endothelial cytoskeleton. Lab Invest. 1991 Aug;65(2):123–137. [PubMed] [Google Scholar]
- Heimark R. L., Twardzik D. R., Schwartz S. M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science. 1986 Sep 5;233(4768):1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- Khachigian L. M., Lindner V., Williams A. J., Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996 Mar 8;271(5254):1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- Kohler T. R., Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler Thromb. 1992 Aug;12(8):963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell. 1980 Nov;22(2 Pt 2):555–561. doi: 10.1016/0092-8674(80)90365-7. [DOI] [PubMed] [Google Scholar]
- Ku D. N., Giddens D. P., Zarins C. K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985 May-Jun;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Adamson S. L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res. 1981 Apr;48(4):481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Bendeck M. P., Keeley F. W. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989 Apr;256(4 Pt 2):H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Graham J. J., Kim D., Gotlieb A. I. Dynamics of shear-induced redistribution of F-actin in endothelial cells in vivo. Arterioscler Thromb. 1991 Nov-Dec;11(6):1814–1820. doi: 10.1161/01.atv.11.6.1814. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Reidy M. A., Kline R. L. Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. Arteriosclerosis. 1986 Mar-Apr;6(2):146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Haudenschild C. C. Junctional transfer in wounded cultures of bovine aortic endothelial cells. Lab Invest. 1988 Sep;59(3):373–379. [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Expression of basic fibroblast growth factor and its receptor by smooth muscle cells and endothelium in injured rat arteries. An en face study. Circ Res. 1993 Sep;73(3):589–595. doi: 10.1161/01.res.73.3.589. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F. Imbalance of endothelium-derived relaxing and contracting factors. A new concept in hypertension? Am J Hypertens. 1990 Apr;3(4):317–330. doi: 10.1093/ajh/3.4.317. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel T., Resnick N., Atkinson W. J., Dewey C. F., Jr, Gimbrone M. A., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994 Aug;94(2):885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott M. F., Müller K. R. Endothelial regeneration in hypertensive and genetically hypercholesterolemic rats. Arteriosclerosis. 1983 May-Jun;3(3):206–214. doi: 10.1161/01.atv.3.3.206. [DOI] [PubMed] [Google Scholar]
- Ramsay M. M., Walker L. N., Bowyer D. E. Narrow superficial injury to rabbit aortic endothelium. The healing process as observed by scanning electron microscopy. Atherosclerosis. 1982 Jun;43(2-3):233–243. doi: 10.1016/0021-9150(82)90025-9. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Remuzzi A., Dewey C. F., Jr, Davies P. F., Gimbrone M. A., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21(4):617–630. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- Rogers K. A., McKee N. H., Kalnins V. I. Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc Natl Acad Sci U S A. 1985 May;82(10):3272–3276. doi: 10.1073/pnas.82.10.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Reidy M. A., Selden S. C., 3rd, Haudenschild C. C. Maintenance of integrity in aortic endothelium. Fed Proc. 1980 Jul;39(9):2618–2625. [PubMed] [Google Scholar]
- Uematsu M., Kitabatake A., Tanouchi J., Doi Y., Masuyama T., Fujii K., Yoshida Y., Ito H., Ishihara K., Hori M. Reduction of endothelial microfilament bundles in the low-shear region of the canine aorta. Association with intimal plaque formation in hypercholesterolemia. Arterioscler Thromb. 1991 Jan-Feb;11(1):107–115. doi: 10.1161/01.atv.11.1.107. [DOI] [PubMed] [Google Scholar]
- Walpola P. L., Gotlieb A. I., Cybulsky M. I., Langille B. L. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995 Jan;15(1):2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- Walpola P. L., Gotlieb A. I., Langille B. L. Monocyte adhesion and changes in endothelial cell number, morphology, and F-actin distribution elicited by low shear stress in vivo. Am J Pathol. 1993 May;142(5):1392–1400. [PMC free article] [PubMed] [Google Scholar]
- Wechezak A. R., Wight T. N., Viggers R. F., Sauvage L. R. Endothelial adherence under shear stress is dependent upon microfilament reorganization. J Cell Physiol. 1989 Apr;139(1):136–146. doi: 10.1002/jcp.1041390120. [DOI] [PubMed] [Google Scholar]
- White G. E., Fujiwara K. Expression and intracellular distribution of stress fibers in aortic endothelium. J Cell Biol. 1986 Jul;103(1):63–70. doi: 10.1083/jcb.103.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. In vitro reendothelialization of a single-cell wound. Role of microfilament bundles in rapid lamellipodia-mediated wound closure. Lab Invest. 1984 Jul;51(1):75–81. [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. The reorganization of microfilaments, centrosomes, and microtubules during in vitro small wound reendothelialization. J Cell Biol. 1988 Nov;107(5):1777–1783. doi: 10.1083/jcb.107.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand T., Majno G., Nunnari J. J., Hoffman A. H., Savilonis B. J., MacWilliams B., Joris I. Lipid deposition and intimal stress and strain. A study in rats with aortic stenosis. Am J Pathol. 1991 Jul;139(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- Zarins C. K., Zatina M. A., Giddens D. P., Ku D. N., Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987 Mar;5(3):413–420. [PubMed] [Google Scholar]