Abstract
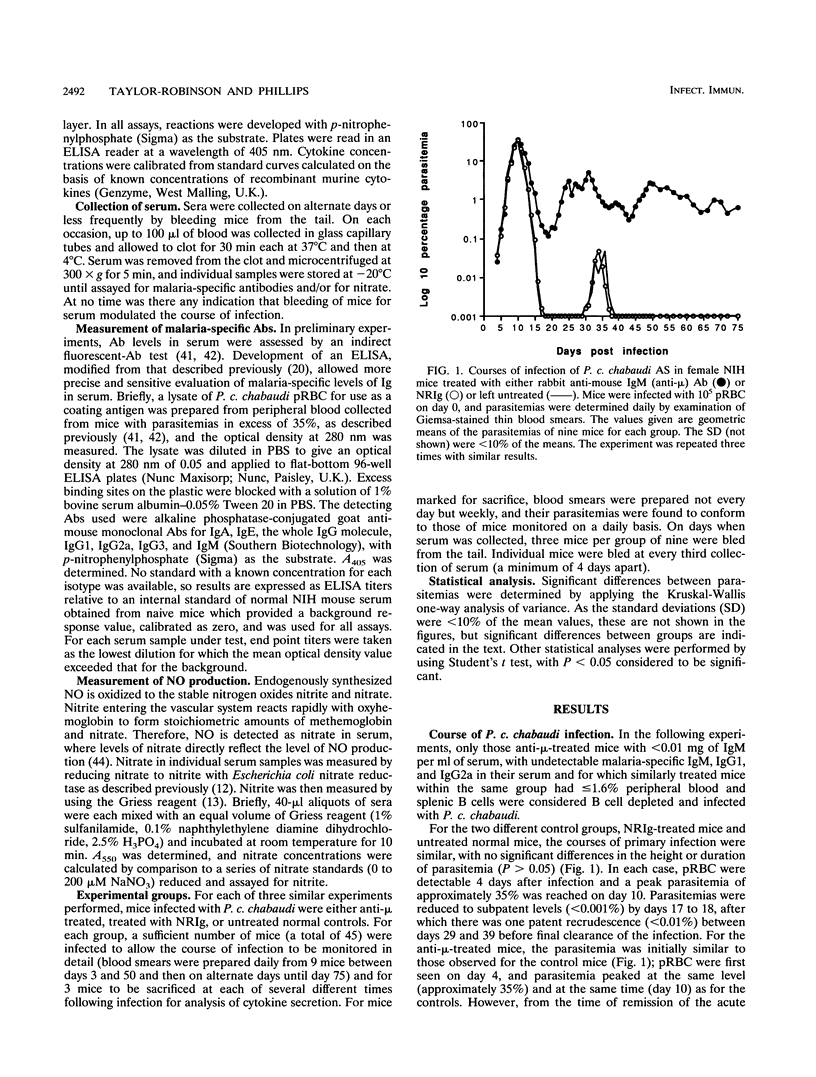
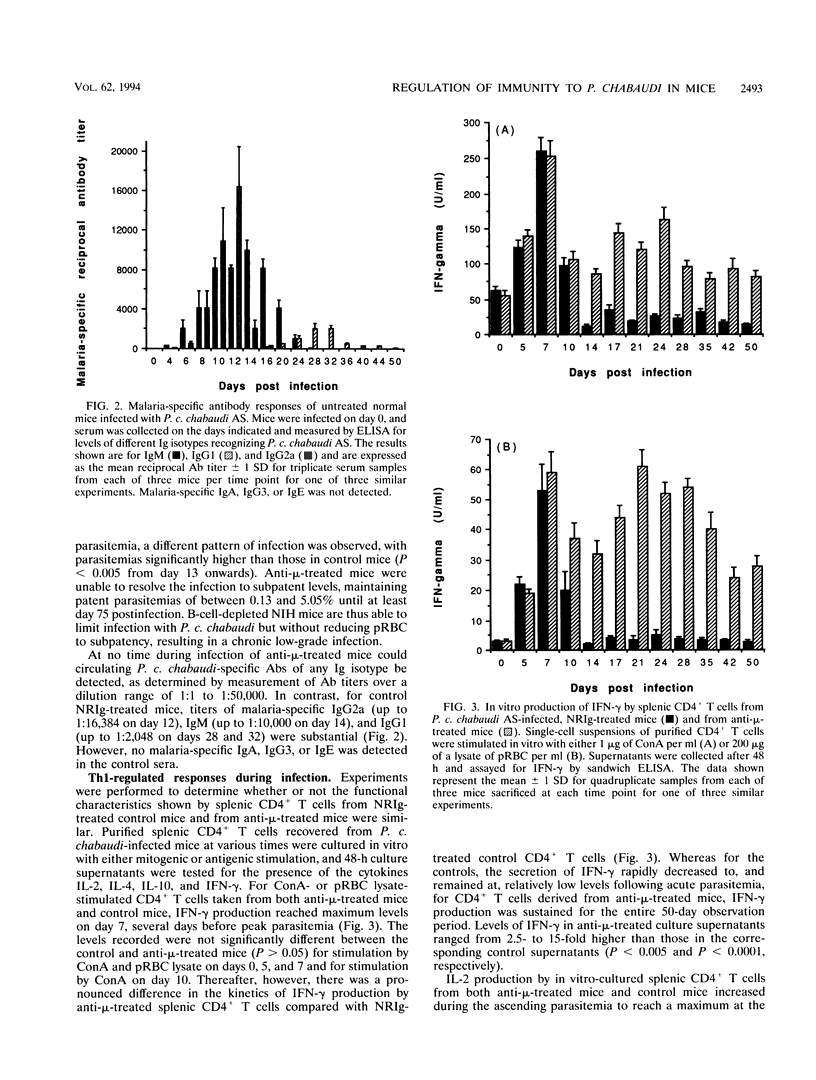
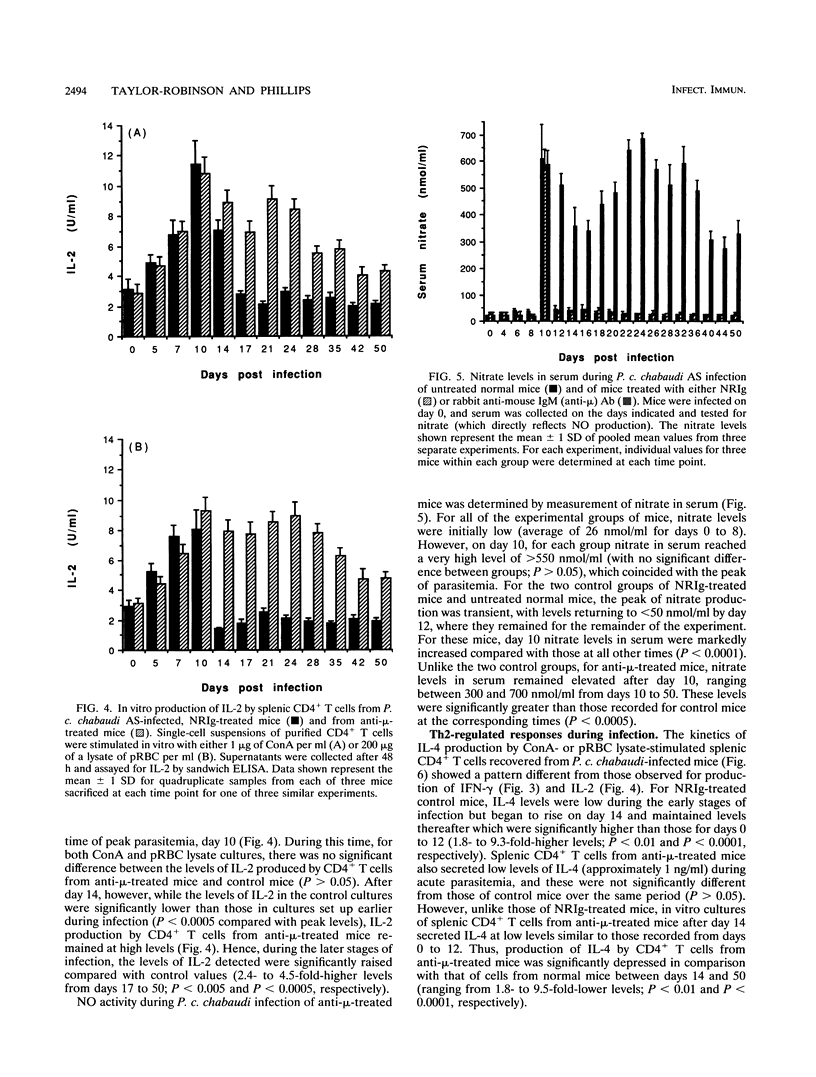
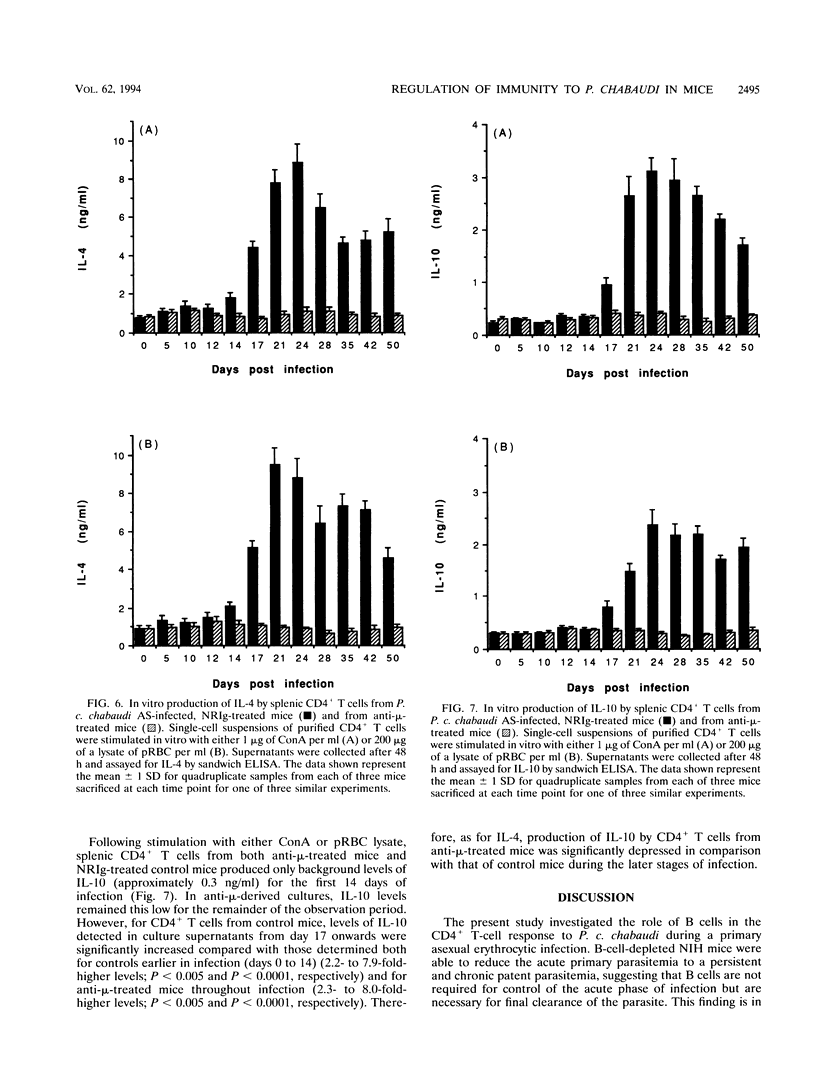
The induction of T-helper cell subsets during the course of blood stage Plasmodium chabaudi chabaudi infection was compared in immunologically intact NIH mice and mice that were depleted of B cells from birth by treatment with anti-mu antibodies. For intact mice, in which the acute primary parasitemia peaked 10 days following infection, purified splenic CD4+ T cells recovered during the ascending parasitemia produced high levels in vitro of interleukin 2 (IL-2) (peak levels on day 10) and gamma interferon (IFN-gamma) (peak levels on day 7). Sera collected from these mice at around this time contained relatively high levels of P. c. chabaudi-specific immunoglobulin 2a (peak levels on day 12), and serum nitric oxide activity was significantly elevated at peak parasitemia. During the descending primary parasitemia, production of IFN-gamma and IL-2 decreased, while levels of IL-4 and IL-10 produced by splenic CD4+ T cells were significantly raised from the time at which subpatency was recorded (day 17) and persisted for at least 50 days. This was concomitant with a significant increase in levels of parasite-specific immunoglobulin G1, which peaked at around the time of recrudescence. Thus, in normal mice, sequential appearance of Th1 and Th2 responses was observed. In contrast, in B-cell-depleted mice, recovery from acute primary parasitemia was followed by a persistent patent infection which did not drop below 0.1% for at least 75 days after initiation of infection. These mice were unable to mount a significant Th2 response, manifest as an enduring inability of splenic CD4+ T cells to produce significant levels of IL-4 and IL-10. IL-2 and IFN-gamma levels remained significantly elevated throughout the 50-day observation period, and there was sustained production of nitric oxide. These data show that immune responses mediated by CD4+ T cells of the Th1 subset are capable of limiting infection beyond the initial acute phase, but that they do not eliminate parasitemia. Furthermore, as the progression from a Th1-regulated to a Th2-regulated immune response fails to occur in B-cell-depleted mice, the data suggest that B cells are required for the downregulation of Th1-mediated and/or the generation of Th2-mediated protective immunity to P. c. chabaudi.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abehsira-Amar O., Gibert M., Joliy M., Thèze J., Jankovic D. L. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992 Jun 15;148(12):3820–3829. [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher P. A., Wei G., Menon J. N., Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992 Jul 24;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- DeKruyff R. H., Fang Y., Umetsu D. T. IL-4 synthesis by in vivo primed keyhole limpet hemocyanin-specific CD4+ T cells. I. Influence of antigen concentration and antigen-presenting cell type. J Immunol. 1992 Dec 1;149(11):3468–3476. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HayGlass K. T., Naides S. J., Benacerraf B., Sy M. S. T cell development in B cell deficient mice. III. Restriction specificity of suppressor T cell factor(s) produced in mice treated chronically with rabbit anti-mouse mu chain antibody. J Mol Cell Immunol. 1985;2(2):107–117. [PubMed] [Google Scholar]
- Hayglass K. T., Naides S. J., Scott C. F., Jr, Benacerraf B., Sy M. S. T cell development in B cell-deficient mice. IV. The role of B cells as antigen-presenting cells in vivo. J Immunol. 1986 Feb 1;136(3):823–829. [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Evans C. B., Asofsky R., Taylor D. W. Immunoglobulin isotype distribution of malaria-specific antibodies produced during infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 1984 Sep;87(2):452–461. doi: 10.1016/0008-8749(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Gillard S., Simon B., Slade S., Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1(4):416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989 Nov;5(11):362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Meding S. J., Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol. 1991 Jun;21(6):1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Podoba J. E., Stevenson M. M. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991 Jan;59(1):51–58. doi: 10.1128/iai.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Schmitz J., Assenmacher M., Radbruch A. Regulation of T helper cell cytokine expression: functional dichotomy of antigen-presenting cells. Eur J Immunol. 1993 Jan;23(1):191–199. doi: 10.1002/eji.1830230130. [DOI] [PubMed] [Google Scholar]
- Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993 Apr 23;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- Scott P. Selective differentiation of CD4+ T helper cell subsets. Curr Opin Immunol. 1993 Jun;5(3):391–397. doi: 10.1016/0952-7915(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine G. H., Jimmo B. L., Ianelli C., Jarvis L. Strong priming of T cells adoptively transferred into scid mice. J Exp Med. 1991 Dec 1;174(6):1653–1656. doi: 10.1084/jem.174.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Bradley L. M., Croft M., Tonkonogy S., Atkins G., Weinberg A. D., Duncan D. D., Hedrick S. M., Dutton R. W., Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991 Oct;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Süss G., Eichmann K., Kury E., Linke A., Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988 Dec;56(12):3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S. Functional characterization of protective CD4+ T-cell clones reactive to the murine malaria parasite Plasmodium chabaudi. Immunology. 1992 Sep;77(1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S. Protective CD4+ T-cell lines raised against Plasmodium chabaudi show characteristics of either Th1 or Th2 cells. Parasite Immunol. 1993 Jun;15(6):301–310. doi: 10.1111/j.1365-3024.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S., Severn A., Moncada S., Liew F. Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993 Jun 25;260(5116):1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S. Th1 and Th2 CD4+ T cell clones specific for Plasmodium chabaudi but not for an unrelated antigen protect against blood stage P. chabaudi infection. Eur J Immunol. 1994 Jan;24(1):158–164. doi: 10.1002/eji.1830240124. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid T., Langhorne J. Altered response of CD4+ T cell subsets to Plasmodium chabaudi chabaudi in B cell-deficient mice. Int Immunol. 1993 Oct;5(10):1343–1348. doi: 10.1093/intimm/5.10.1343. [DOI] [PubMed] [Google Scholar]



