Abstract
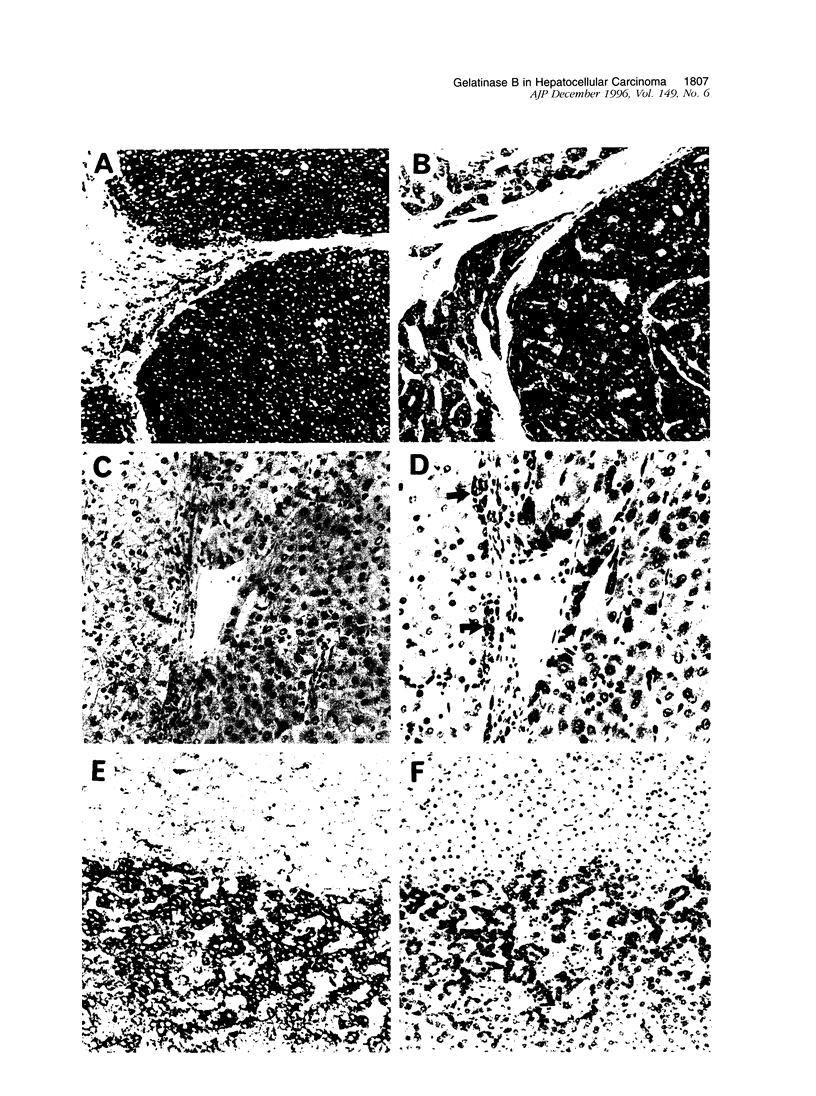
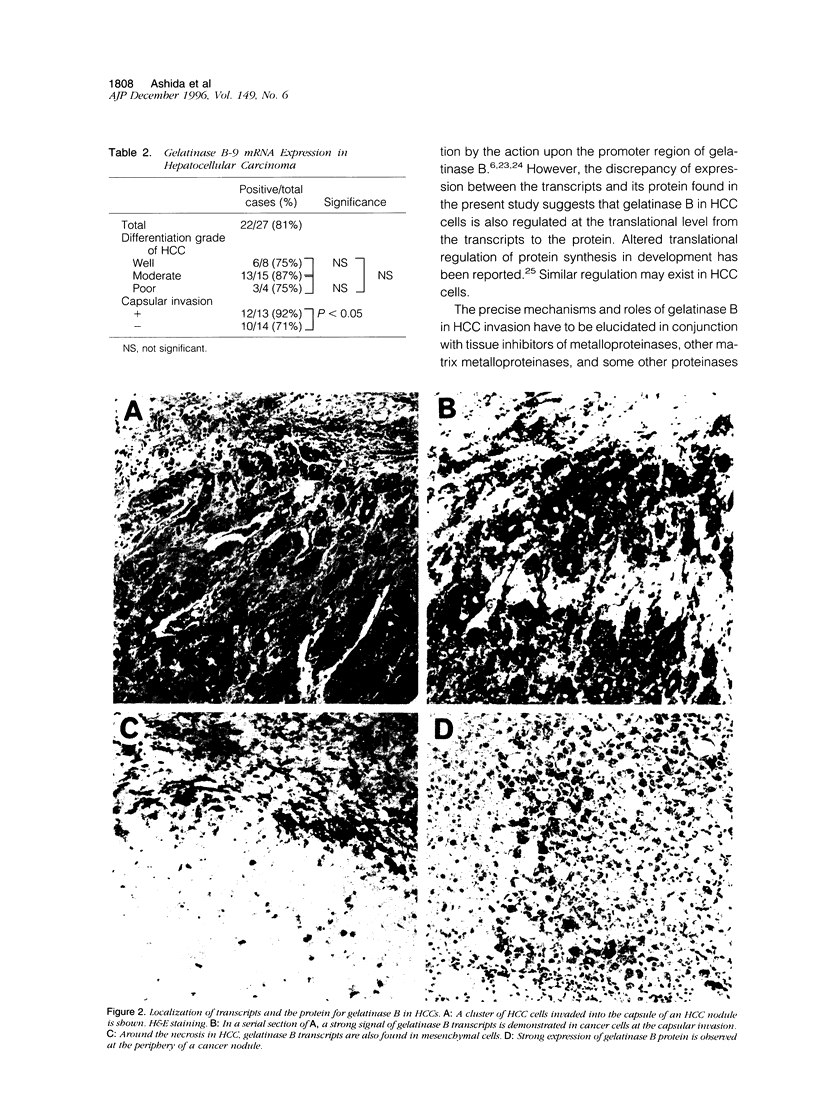
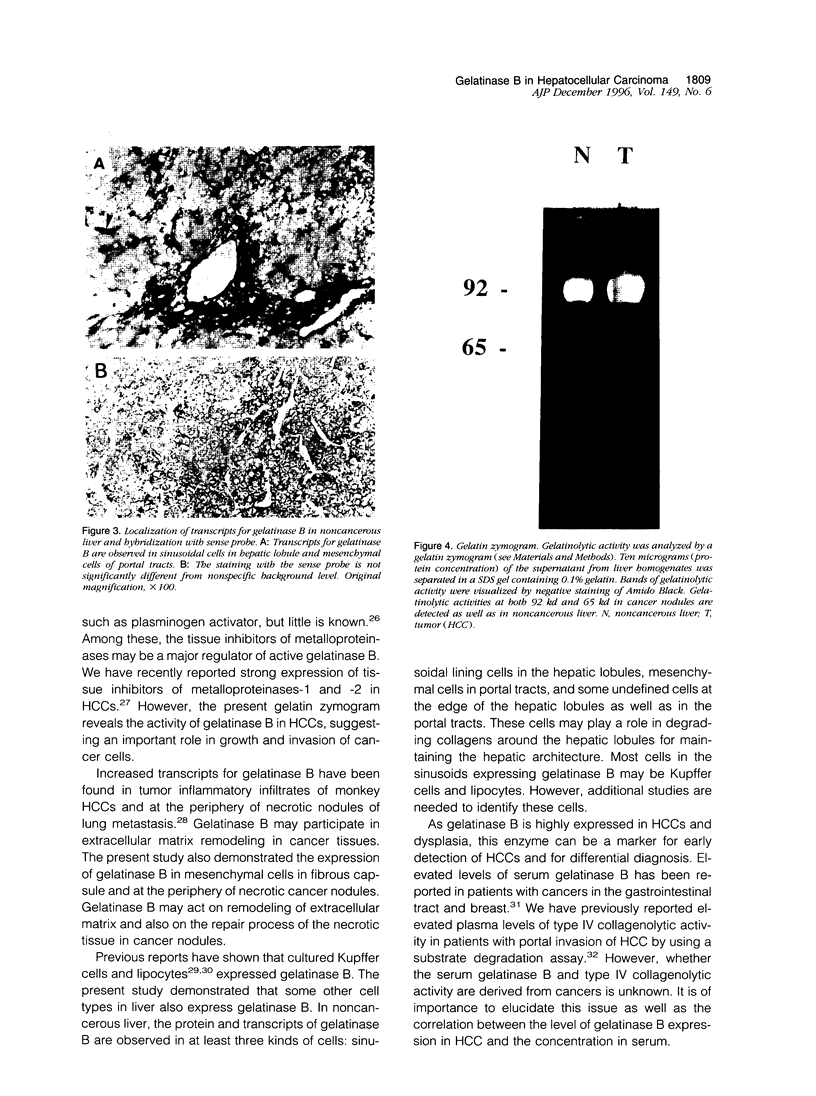
To examine the possible involvement of gelatinase B in human hepatocellular carcinoma (HCC), cellular localization of transcripts and protein of gelatinase B were studied by using in situ hybridization and immunohistochemistry. Transcripts for gelatinase B were observed in tumor cells in 22 cases of 27 HCCs and also in dysplastic nodules. However, there was no significant difference in the expression among histological grades of HCC. The expression was mostly homogeneous, but the intensity varied with the nodules. Of 13 cases with capsular invasion, 12 expressed gelatinase B, whereas 10 of 14 without capsular invasion did (P < 0.05). Gelatinase B transcripts were commonly observed in the sinusoidal cells of the hepatic lobules, in mesenchymal cells both in fibrous capsules and around the necrosis, and also in some undefined cells of the portal tracts of noncancerous liver. Localization of gelatinase B protein was mostly similar to but sometimes different from that of the transcripts in cancer nodules. In conclusion, the expression of gelatinase B appears to be an important characteristic of malignant transformation of hepatocytes. The findings suggest that gelatinase B synthesized by cancer cells plays an important role in the growth and invasion of HCC by degrading surrounding extracellular matrices.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Friedman S. L., Roll F. J., Bissell D. M. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest. 1989 Oct;84(4):1076–1085. doi: 10.1172/JCI114270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canete-Soler R., Litzky L., Lubensky I., Muschel R. J. Localization of the 92 kd gelatinase mRNA in squamous cell and adenocarcinomas of the lung using in situ hybridization. Am J Pathol. 1994 Mar;144(3):518–527. [PMC free article] [PubMed] [Google Scholar]
- Curtis D., Lehmann R., Zamore P. D. Translational regulation in development. Cell. 1995 Apr 21;81(2):171–178. doi: 10.1016/0092-8674(95)90325-9. [DOI] [PubMed] [Google Scholar]
- Gress T. M., Müller-Pillasch F., Lerch M. M., Friess H., Büchler M., Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995 Aug 9;62(4):407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- Grigioni W. F., Garbisa S., D'Errico A., Baccarini P., Stetler-Stevenson W. G., Liotta L. A., Mancini A. M. Evaluation of hepatocellular carcinoma aggressiveness by a panel of extracellular matrix antigens. Am J Pathol. 1991 Mar;138(3):647–654. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., Kobayashi M., Tsuji T. Serum type IV collagen-degrading enzyme in hepatocellular carcinoma with metastasis. Acta Med Okayama. 1988 Feb;42(1):1–6. doi: 10.18926/AMO/31039. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauricella-Lefebvre M. A., Castronovo V., Sato H., Seiki M., French D. L., Merville M. P. Stimulation of the 92-kD type IV collagenase promoter and enzyme expression in human melanoma cells. Invasion Metastasis. 1993;13(6):289–300. [PubMed] [Google Scholar]
- Lindsay C. K., Thorgeirsson U. P. Localization of messenger RNA for tissue inhibitor of metalloproteinases-1 and type IV collagenases/gelatinases in monkey hepatocellular carcinomas. Clin Exp Metastasis. 1995 Sep;13(5):381–388. doi: 10.1007/BF00121914. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Hartzler J. L., Pelina M. D., Thorgeirsson U. P. Studies on the ability of 65-kDa and 92-kDa tumor cell gelatinases to degrade type IV collagen. J Biol Chem. 1990 Dec 15;265(35):21929–21934. [PubMed] [Google Scholar]
- Mainardi C. L., Hibbs M. S., Hasty K. A., Seyer J. M. Purification of a type V collagen degrading metalloproteinase from rabbit alveolar macrophages. Coll Relat Res. 1984 Dec;4(6):479–492. doi: 10.1016/s0174-173x(84)80014-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa H., Ashida K., Higashi T., Ohguchi S., Tsuboi S., Hino N., Nouso K., Urabe Y., Kinugasa N., Yoshida K. Cellular distribution of transcripts for tissue inhibitor of metalloproteinases 1 and 2 in human hepatocellular carcinomas. Hepatology. 1996 Jul;24(1):82–88. doi: 10.1053/jhep.1996.v24.pm0008707287. [DOI] [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Davies B. D., Balkwill F. R. Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer. 1994 Jul 1;58(1):50–56. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P. B., Simsir A., Valea F., French D. L. Correlation of the in situ detection of polymerase chain reaction-amplified metalloproteinase complementary DNAs and their inhibitors with prognosis in cervical carcinoma. Cancer Res. 1995 Jan 15;55(2):267–275. [PubMed] [Google Scholar]
- Okada Y., Gonoji Y., Naka K., Tomita K., Nakanishi I., Iwata K., Yamashita K., Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992 Oct 25;267(30):21712–21719. [PubMed] [Google Scholar]
- Okada Y., Tsuchiya H., Shimizu H., Tomita K., Nakanishi I., Sato H., Seiki M., Yamashita K., Hayakawa T. Induction and stimulation of 92-kDa gelatinase/type IV collagenase production in osteosarcoma and fibrosarcoma cell lines by tumor necrosis factor alpha. Biochem Biophys Res Commun. 1990 Sep 14;171(2):610–617. doi: 10.1016/0006-291x(90)91190-4. [DOI] [PubMed] [Google Scholar]
- Okuda K., Musha H., Nakajima Y., Kubo Y., Shimokawa Y., Nagasaki Y., Sawa Y., Jinnouchi S., Kaneko T., Obata H. Clinicopathologic features of encapsulated hepatocellular carcinoma: a study of 26 cases. Cancer. 1977 Sep;40(3):1240–1245. doi: 10.1002/1097-0142(197709)40:3<1240::aid-cncr2820400339>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Huhtala P., Hurskainen T., Danø K., Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992 Mar 1;52(5):1336–1341. [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Tryggvason K., Danø K. Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol. 1993 Feb;142(2):359–365. [PMC free article] [PubMed] [Google Scholar]
- Rojkind M., Giambrone M. A., Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979 Apr;76(4):710–719. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Soini Y., Autio-Harmainen H. Synthesis and degradation of basement membranes in benign and malignant salivary gland tumours. A study by in situ hybridization. J Pathol. 1993 Jul;170(3):291–296. doi: 10.1002/path.1711700312. [DOI] [PubMed] [Google Scholar]
- Soini Y., Hurskainen T., Höyhtyä M., Oikarinen A., Autio-Harmainen H. 72 KD and 92 KD type IV collagenase, type IV collagen, and laminin mRNAs in breast cancer: a study by in situ hybridization. J Histochem Cytochem. 1994 Jul;42(7):945–951. doi: 10.1177/42.7.8014478. [DOI] [PubMed] [Google Scholar]
- Torimura T., Ueno T., Inuzuka S., Tanaka M., Abe H., Tanikawa K. Mechanism of fibrous capsule formation surrounding hepatocellular carcinoma. Immunohistochemical study. Arch Pathol Lab Med. 1991 Apr;115(4):365–371. [PubMed] [Google Scholar]
- Watanabe H., Nakanishi I., Yamashita K., Hayakawa T., Okada Y. Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci. 1993 Apr;104(Pt 4):991–999. doi: 10.1242/jcs.104.4.991. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Winwood P. J., Schuppan D., Iredale J. P., Kawser C. A., Docherty A. J., Arthur M. J. Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology. 1995 Jul;22(1):304–315. [PubMed] [Google Scholar]
- Yamanaka N., Okamoto E., Toyosaka A., Mitunobu M., Fujihara S., Kato T., Fujimoto J., Oriyama T., Furukawa K., Kawamura E. Prognostic factors after hepatectomy for hepatocellular carcinomas. A univariate and multivariate analysis. Cancer. 1990 Mar 1;65(5):1104–1110. doi: 10.1002/1097-0142(19900301)65:5<1104::aid-cncr2820650511>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Zucker S., Lysik R. M., Zarrabi M. H., Moll U. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993 Jan 1;53(1):140–146. [PubMed] [Google Scholar]