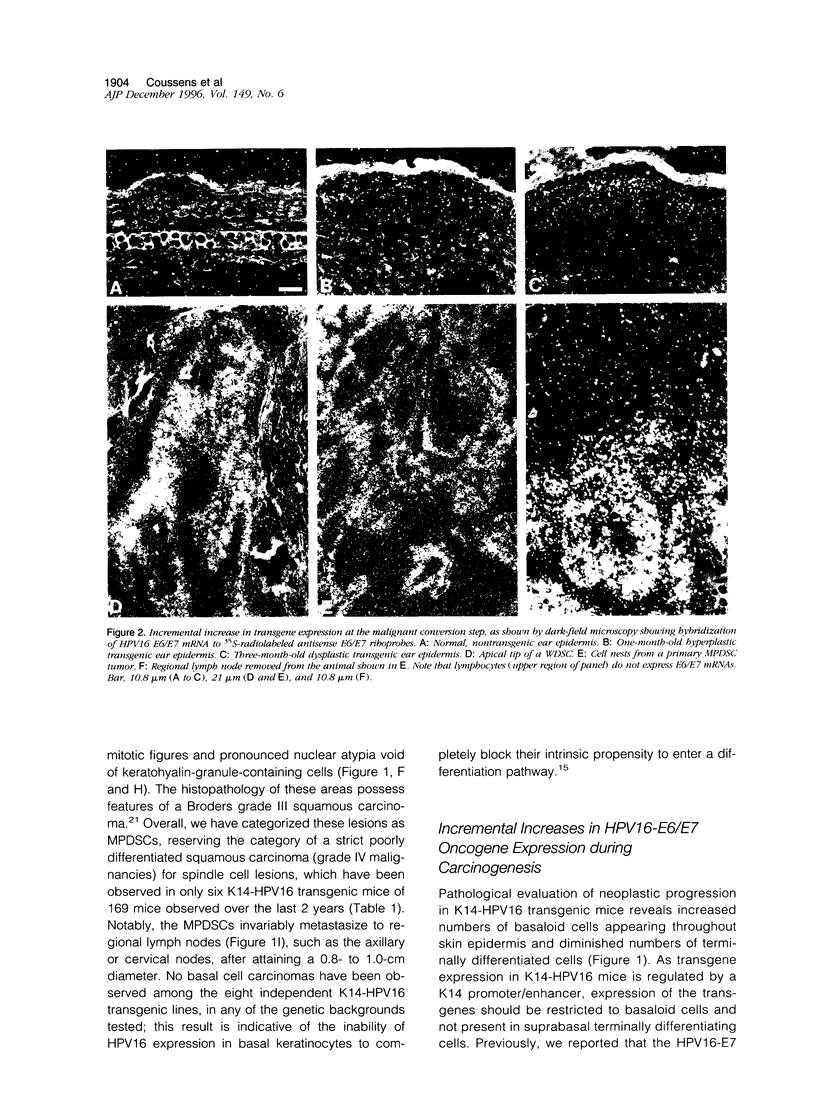
Abstract
Reproducible multi-stage progression to invasive squamous carcinoma of the epidermis has been achieved in transgenic mice expressing the HPV16 early-region genes, including the E6/E7 oncogenes, under the control of the human keratin-14 promoter/enhancer. Although 100% of K14-HPV16 transgenic animals develop hyperplastic and/or dysplastic lesions in several inbred backgrounds, including C57BL/6, BALB/c, and SSIN/SENCAR, only mice backcrossed into the FVB/n background progress to malignant squamous cell carcinomas of two pathological grades, well differentiated and moderate/poorly differentiated (WDSC or MPDSC, respectively), each displaying characteristic patterns of malignant behavior. WDSCs typically arise within the epidermis of the ear and invade deeply into the underlying dermis but fail to metastasize, whereas MPDSCs develop on the chest and truncal skin and invariably metastasize to regional lymph nodes. The transition to the malignant state, in 21% of FVB/n transgenic mice, is characterized by alteration of the repertoire of keratin intermediate filament proteins expressed within neoplastic epidermis, such that WDSCs maintain expression of keratins common to terminally differentiating stratified keratinocytes (K10), whereas MPDSCs are distinguished from WDSCs by activation of embryonic and mucosal keratins (K13, K8, and K19). Precursor hyperplastic and dysplastic lesions are characterized by a progressively increased proliferative index, striking morphological alterations in keratinocyte cell-cell and cell-matrix interactions, and extensive remodeling of the underlying dermal stroma. Remarkably, this extensive stromal remodeling, which may facilitate both angiogenesis and eventual tumor cell invasion, develops early at the dysplastic stage in all animals well before malignant conversion.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apple R. J., Erlich H. A., Klitz W., Manos M. M., Becker T. M., Wheeler C. M. HLA DR-DQ associations with cervical carcinoma show papillomavirus-type specificity. Nat Genet. 1994 Feb;6(2):157–162. doi: 10.1038/ng0294-157. [DOI] [PubMed] [Google Scholar]
- Arbeit J. M., Münger K., Howley P. M., Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994 Jul;68(7):4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit J. M. Transgenic models of epidermal neoplasia and multistage carcinogenesis. Cancer Surv. 1996;26:7–34. [PubMed] [Google Scholar]
- Bosch F. X., Muñoz N., de Sanjosé S., Izarzugaza I., Gili M., Viladiu P., Tormo M. J., Moreo P., Ascunce N., Gonzalez L. C. Risk factors for cervical cancer in Colombia and Spain. Int J Cancer. 1992 Nov 11;52(5):750–758. doi: 10.1002/ijc.2910520514. [DOI] [PubMed] [Google Scholar]
- Brown K., Balmain A. Transgenic mice and squamous multistage skin carcinogenesis. Cancer Metastasis Rev. 1995 Jun;14(2):113–124. doi: 10.1007/BF00665795. [DOI] [PubMed] [Google Scholar]
- Byrne C., Tainsky M., Fuchs E. Programming gene expression in developing epidermis. Development. 1994 Sep;120(9):2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- Casanova L., Bravo A., Were F., Ramírez A., Jorcano J. J., Vidal M. Tissue-specific and efficient expression of the human simple epithelial keratin 8 gene in transgenic mice. J Cell Sci. 1995 Feb;108(Pt 2):811–820. doi: 10.1242/jcs.108.2.811. [DOI] [PubMed] [Google Scholar]
- D'Armiento J., DiColandrea T., Dalal S. S., Okada Y., Huang M. T., Conney A. H., Chada K. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol. 1995 Oct;15(10):5732–5739. doi: 10.1128/mcb.15.10.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter L. M., Stoler M. H., Broker T. R., Chow L. T. Induction of proliferating cell nuclear antigen in differentiated keratinocytes of human papillomavirus-infected lesions. Hum Pathol. 1994 Apr;25(4):343–348. doi: 10.1016/0046-8177(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Eaton S., Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995 Jul 14;82(1):5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Eckert R. L. Structure, function, and differentiation of the keratinocyte. Physiol Rev. 1989 Oct;69(4):1316–1346. doi: 10.1152/physrev.1989.69.4.1316. [DOI] [PubMed] [Google Scholar]
- Garcia R. L., Coltrera M. D., Gown A. M. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989 Apr;134(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Conti I. B., Bianchi A. B., Fischer S. M., Reiners J. J., Jr, Conti C. J., Slaga T. J. Dissociation of sensitivities to tumor promotion and progression in outbred and inbred SENCAR mice. Cancer Res. 1992 Jun 15;52(12):3432–3435. [PubMed] [Google Scholar]
- Hennings H., Glick A. B., Lowry D. T., Krsmanovic L. S., Sly L. M., Yuspa S. H. FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis. 1993 Nov;14(11):2353–2358. doi: 10.1093/carcin/14.11.2353. [DOI] [PubMed] [Google Scholar]
- Hurlin P. J., Foley K. P., Ayer D. E., Eisenman R. N., Hanahan D., Arbeit J. M. Regulation of Myc and Mad during epidermal differentiation and HPV-associated tumorigenesis. Oncogene. 1995 Dec 21;11(12):2487–2501. [PubMed] [Google Scholar]
- Koutsky L. A., Holmes K. K., Critchlow C. W., Stevens C. E., Paavonen J., Beckmann A. M., DeRouen T. A., Galloway D. A., Vernon D., Kiviat N. B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- Kuruc N., Leube R. E., Moll I., Bader B. L., Franke W. W. Synthesis of cytokeratin 13, a component characteristic of internal stratified epithelia, is not induced in human epidermal tumors. Differentiation. 1989 Dec;42(2):111–123. doi: 10.1111/j.1432-0436.1989.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Pan H., Pitot H. C., Liem A., Jackson M., Griep A. E. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Alexander C. M. Use of keratin antibodies in tumor diagnosis. Semin Cancer Biol. 1990 Jun;1(3):165–179. [PubMed] [Google Scholar]
- Leder A., Kuo A., Cardiff R. D., Sinn E., Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leube R. E., Bosch F. X., Romano V., Zimbelmann R., Höfler H., Franke W. W. Cytokeratin expression in simple epithelia. III. Detection of mRNAs encoding human cytokeratins nos. 8 and 18 in normal and tumor cells by hybridization with cDNA sequences in vitro and in situ. Differentiation. 1986;33(1):69–85. doi: 10.1111/j.1432-0436.1986.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G., Steeg P. S. Cancer invasion and metastasis: positive and negative regulatory elements. Cancer Invest. 1991;9(5):543–551. doi: 10.3109/07357909109018952. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Kirnbauer R., Schiller J. T. Genital human papillomavirus infection. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Markey A. C., Lane E. B., Churchill L. J., MacDonald D. M., Leigh I. M. Expression of simple epithelial keratins 8 and 18 in epidermal neoplasia. J Invest Dermatol. 1991 Nov;97(5):763–770. doi: 10.1111/1523-1747.ep12486607. [DOI] [PubMed] [Google Scholar]
- Miracco C., De Santi M. M., Lio R., Biagioli M., Tosi P., Luzi P. Quantitatively evaluated ultrastructural findings can add to the differential diagnosis between keratoacanthoma and well differentiated squamous cell carcinoma. J Submicrosc Cytol Pathol. 1992 Jul;24(3):315–321. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma H. E., Van Der Velden L. A., Manni J. J., Peters H., Link M., Rutter D. J., Ramaekers F. C. Increased expression of cytokeratins 8, 18 and vimentin in the invasion front of mucosal squamous cell carcinoma. J Pathol. 1993 May;170(1):77–86. doi: 10.1002/path.1711700113. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Romanczuk H., Münger K., Huibregtse J. M., Mietz J. A., Howley P. M. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol. 1994;186:83–99. doi: 10.1007/978-3-642-78487-3_5. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H. Epidemiology of cervical human papillomavirus infections. Curr Top Microbiol Immunol. 1994;186:55–81. doi: 10.1007/978-3-642-78487-3_4. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Kinjo M., Fürstenberger G., Winter H. Sequential expression of mRNA-encoded keratin sets in neonatal mouse epidermis: basal cells with properties of terminally differentiating cells. Cell. 1984 May;37(1):159–170. doi: 10.1016/0092-8674(84)90311-8. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M. Strain differences and solvent effects in mouse skin carcinogenesis experiments using carcinogens, tumor initiators and promoters. Prog Exp Tumor Res. 1983;26:85–109. doi: 10.1159/000407254. [DOI] [PubMed] [Google Scholar]
- Smedts F., Ramaekers F., Troyanovsky S., Pruszczynski M., Link M., Lane B., Leigh I., Schijf C., Vooijs P. Keratin expression in cervical cancer. Am J Pathol. 1992 Aug;141(2):497–511. [PMC free article] [PubMed] [Google Scholar]
- Suo Z., Holm R., Nesland J. M. Squamous cell carcinomas, an immunohistochemical and ultrastructural study. Anticancer Res. 1992 Nov-Dec;12(6B):2025–2031. [PubMed] [Google Scholar]
- Sutter C., Strickland J. E., Welty D. J., Yuspa S. H., Winter H., Schweizer J. v-Ha-ras-induced mouse skin papillomas exhibit aberrant expression of keratin K13 as do their 7,12-dimethylbenz[a]anthracene/12-O-tetradecanoylphorbol-13-acetate -induced analogues. Mol Carcinog. 1991;4(6):467–476. doi: 10.1002/mc.2940040610. [DOI] [PubMed] [Google Scholar]
- Tennenbaum T., Yuspa S. H., Grover A., Castronovo V., Sobel M. E., Yamada Y., De Luca L. M. Extracellular matrix receptors and mouse skin carcinogenesis: altered expression linked to appearance of early markers of tumor progression. Cancer Res. 1992 May 15;52(10):2966–2976. [PubMed] [Google Scholar]
- Vassar R., Hutton M. E., Fuchs E. Transgenic overexpression of transforming growth factor alpha bypasses the need for c-Ha-ras mutations in mouse skin tumorigenesis. Mol Cell Biol. 1992 Oct;12(10):4643–4653. doi: 10.1128/mcb.12.10.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis--thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1994 Mar 1;54(5):1178–1189. [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]