Abstract
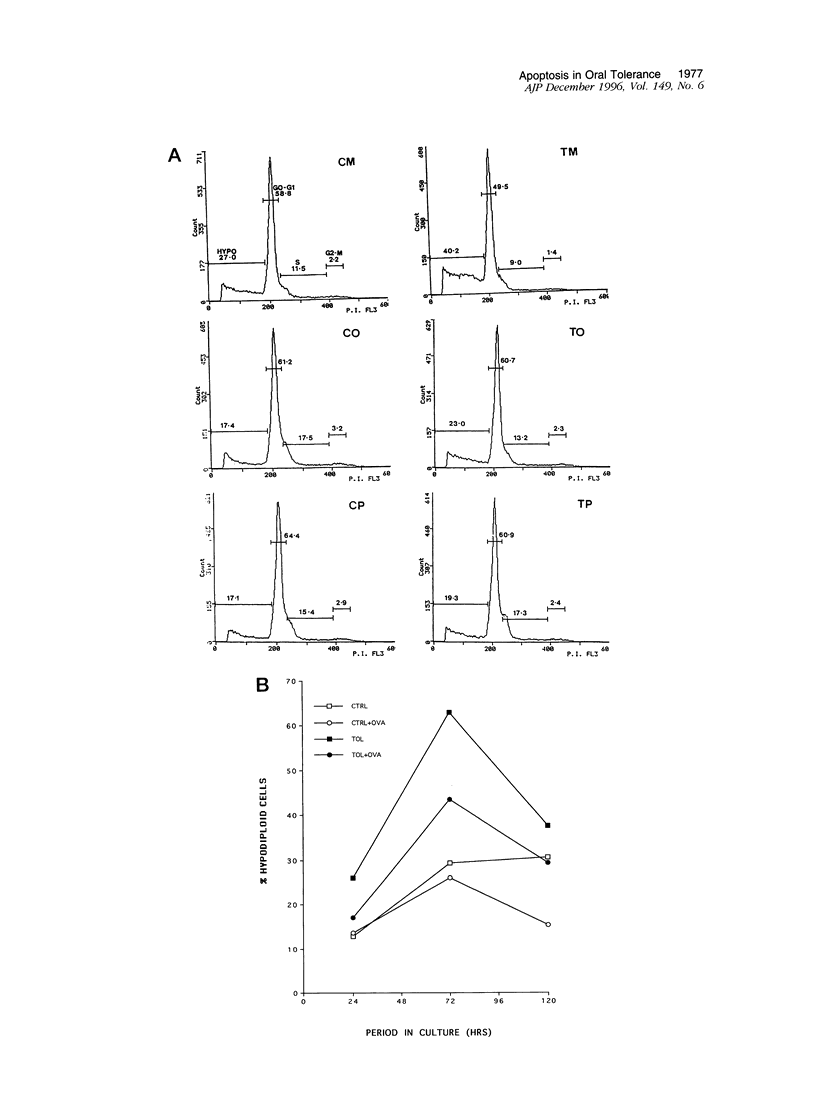
The mechanism responsible for the induction of immunological tolerance by oral administration of soluble antigen remains unclear. Here we show that, when cultured in vitro in the absence of antigen, lymphocytes from mice tolerized with a single feed of 25 mg of ovalbumin display an enhanced mortality in comparison with cells from immunized control animals. This increased cell death affects both CD4+ and CD8+ T-lymphocyte subsets, and morphological and flow cytometric analyses suggest that it occurs via apoptosis. All of the changes associated with the propensity of tolerant cells to die by apoptosis in vitro are reduced by the inclusion of the tolerizing antigen in the cultures. These results suggest that tolerance to dietary proteins is accompanied by functional changes in T lymphocytes that render them susceptible to apoptosis. This mechanism may underlie the profound and permanent tolerance to food antigens found under physiological conditions and may provide a useful basis for immunotherapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baschieri S., Lees R. K., Lussow A. R., MacDonald H. R. Clonal anergy to staphylococcal enterotoxin B in vivo: selective effects on T cell subsets and lymphokines. Eur J Immunol. 1993 Oct;23(10):2661–2666. doi: 10.1002/eji.1830231041. [DOI] [PubMed] [Google Scholar]
- Chen Y., Inobe J., Marks R., Gonnella P., Kuchroo V. K., Weiner H. L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995 Jul 13;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Fishman-Lobell J., Friedman A., Weiner H. L. Different kinetic patterns of cytokine gene expression in vivo in orally tolerant mice. Eur J Immunol. 1994 Nov;24(11):2720–2724. doi: 10.1002/eji.1830241122. [DOI] [PubMed] [Google Scholar]
- Garside P., Steel M., Liew F. Y., Mowat A. M. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1995 Mar;7(3):501–504. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- Garside P., Steel M., Worthey E. A., Satoskar A., Alexander J., Bluethmann H., Liew F. Y., Mowat A. M. T helper 2 cells are subject to high dose oral tolerance and are not essential for its induction. J Immunol. 1995 Jun 1;154(11):5649–5655. [PubMed] [Google Scholar]
- Kearney E. R., Pape K. A., Loh D. Y., Jenkins M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994 Jul;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Oral tolerance. Eating your way towards immunosuppression. Curr Biol. 1994 Feb 1;4(2):178–181. doi: 10.1016/s0960-9822(94)00044-8. [DOI] [PubMed] [Google Scholar]
- Maloy K. J., Mowat A. M., Zamoyska R., Crispe I. N. Phenotypic heterogeneity of intraepithelial T lymphocytes from mouse small intestine. Immunology. 1991 Apr;72(4):555–562. [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Friedman A. In vivo tolerization of Th1 lymphocytes following a single feeding with ovalbumin: anergy in the absence of suppression. Eur J Immunol. 1994 Sep;24(9):1974–1981. doi: 10.1002/eji.1830240906. [DOI] [PubMed] [Google Scholar]
- Miller A., al-Sabbagh A., Santos L. M., Das M. P., Weiner H. L. Epitopes of myelin basic protein that trigger TGF-beta release after oral tolerization are distinct from encephalitogenic epitopes and mediate epitope-driven bystander suppression. J Immunol. 1993 Dec 15;151(12):7307–7315. [PubMed] [Google Scholar]
- Rocha B., Tanchot C., Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993 May 1;177(5):1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C. D., Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994 Nov 3;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Mackin G. A., Matsui M., Orav E. J., Khoury S. J., Dawson D. M., Hafler D. A. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993 Feb 26;259(5099):1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]