Abstract
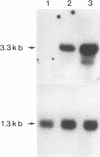
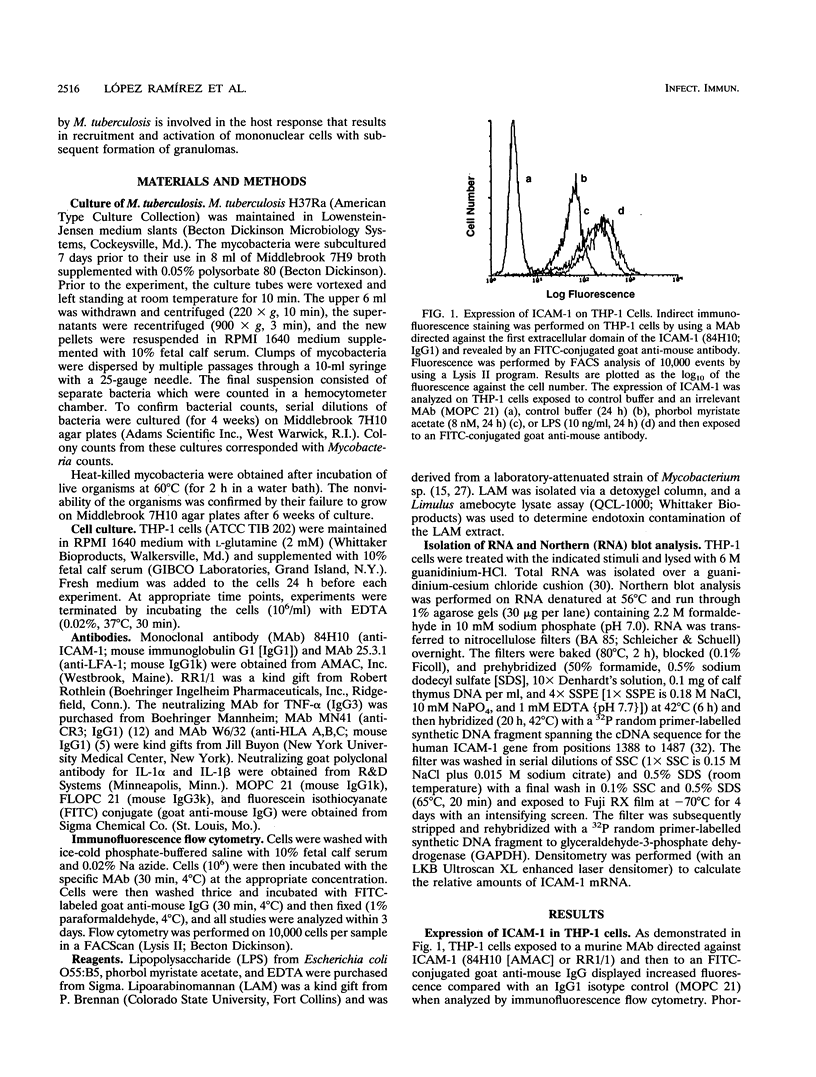
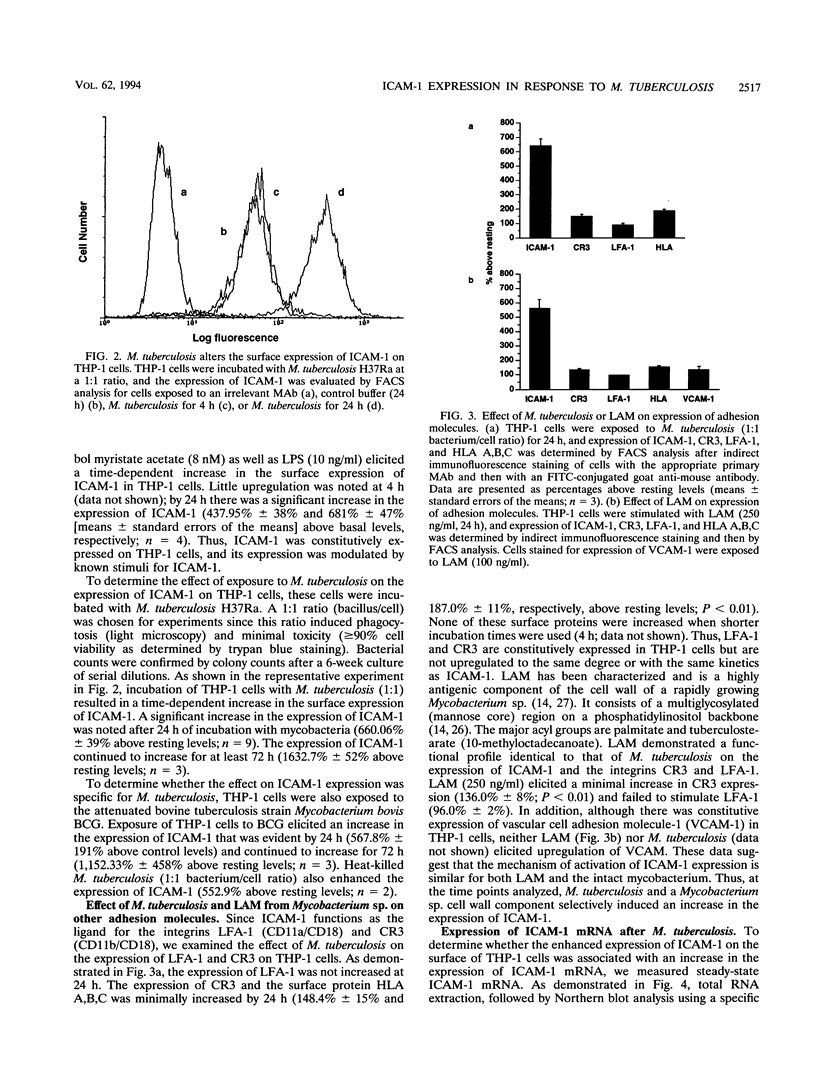
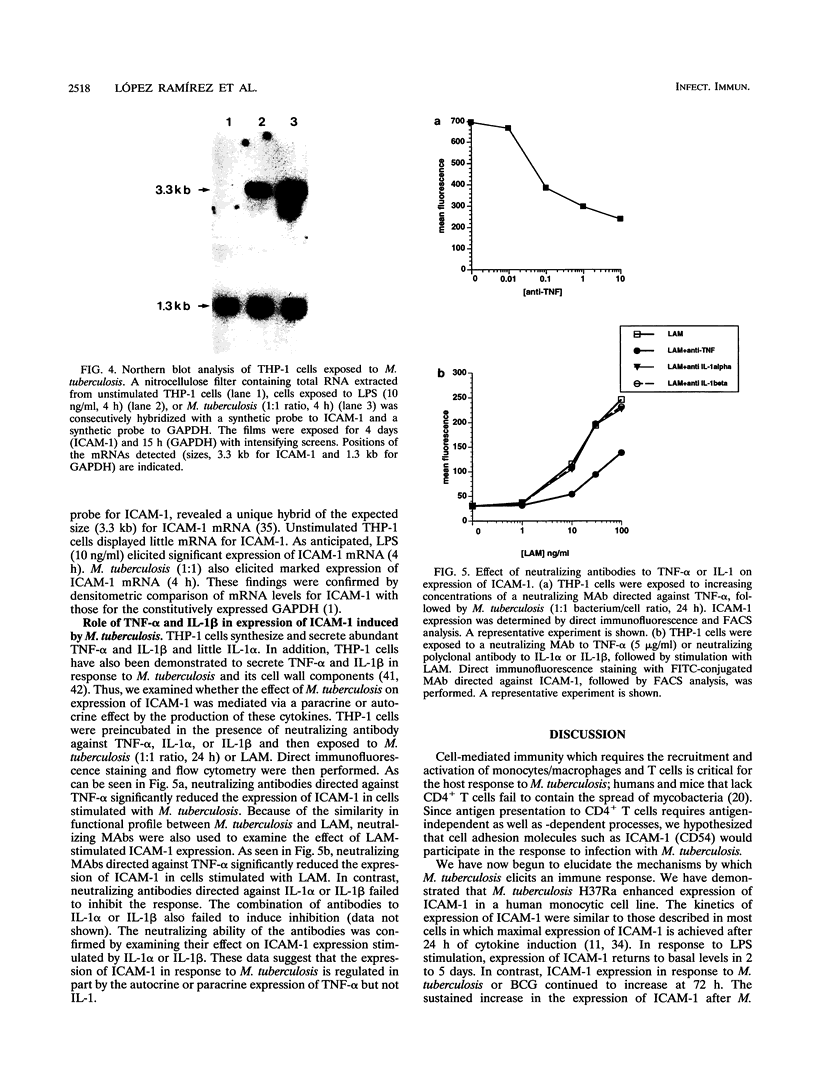
The host response to Mycobacterium tuberculosis is characterized by interactions between mononuclear cells, with recruitment and fusion of these cells culminating in granuloma formation. In addition, the host response to M. tuberculosis requires CD4+ T-cell reactivity, mediated by antigen-independent as well as antigen-dependent mechanisms. Thus, we hypothesized that cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1; CD54) would participate in the response to infection with M. tuberculosis. Exposure of THP-1 cells derived from a monocyte/macrophage cell line to M. tuberculosis (1:1 bacterium/cell ratio) elicited a sustained increase (660% +/- 49% above resting level) in the expression of ICAM-1 that continued for at least 72 h. Neither the expression of vascular cell adhesion molecule 1 (VCAM-1; CD106) nor that of the integrins lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18) or CR3 (CD11b/CD18) was increased to a similar extent at corresponding time points. The increase in ICAM-1 protein expression was accompanied by an increase in steady-state mRNA (Northern [RNA] analysis). Neutralizing monoclonal antibodies directed against tumor necrosis factor alpha but not interleukin 1 alpha or interleukin 1 beta substantially abrogated the response to M. tuberculosis consistent with a paracrine or autocrine response. Continuous upregulation of the expression of ICAM-1 on mononuclear phagocytes induced by M. tuberculosis may mediate the recruitment of monocytes and enhance the antigen presentation of M. tuberculosis, thus permitting the generation and maintenance of the host response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. II. The effect of Mycobacterium tuberculosis. Am J Pathol. 1975 Jul;80(1):101–116. [PMC free article] [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Otterness I. G., Higashi G. I., Forsch C. S., Kunkel S. L. Monokine production by hypersensitivity (Schistosoma mansoni egg) and foreign body (Sephadex bead)-type granuloma macrophages. Evidence for sequential production of IL-1 and tumor necrosis factor. J Immunol. 1989 Feb 15;142(4):1281–1286. [PubMed] [Google Scholar]
- Cybulsky M. I., Fries J. W., Williams A. J., Sultan P., Davis V. M., Gimbrone M. A., Jr, Collins T. Alternative splicing of human VCAM-1 in activated vascular endothelium. Am J Pathol. 1991 Apr;138(4):815–820. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Fries J. W., Williams A. J., Sultan P., Eddy R., Byers M., Shows T., Gimbrone M. A., Jr, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7859–7863. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O., Emilie D., Peuchmaur M., Crevon M. C., D'Agay M. F., Galanaud P. Production of cytokines in sarcoid lymph nodes: preferential expression of interleukin-1 beta and interferon-gamma genes. Hum Pathol. 1992 Mar;23(3):317–323. doi: 10.1016/0046-8177(92)90114-i. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Eddy A., Newman S. L., Cosio F., LeBien T., Michael A. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984 Jun;31(3):371–389. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990 Jun 5;265(16):9272–9279. [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Strieter R. M., Lynch J. P., Remick D. G. Cellular and molecular aspects of granulomatous inflammation. Am J Respir Cell Mol Biol. 1989 Dec;1(6):439–447. doi: 10.1165/ajrcmb/1.6.439. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Melis M., Gjomarkaj M., Pace E., Malizia G., Spatafora M. Increased expression of leukocyte function associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) by alveolar macrophages of patients with pulmonary sarcoidosis. Chest. 1991 Oct;100(4):910–916. doi: 10.1378/chest.100.4.910. [DOI] [PubMed] [Google Scholar]
- Moreno C., Mehlert A., Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988 Nov;74(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Squires K. E., Miralles C. D., Stoeckle M. Y., Granger A. M., Granelli-Piperno A., Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992 Mar 15;148(6):1858–1863. [PubMed] [Google Scholar]
- Möst J., Neumayer H. P., Dierich M. P. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-gamma and expression of LFA-1. Eur J Immunol. 1990 Aug;20(8):1661–1667. doi: 10.1002/eji.1830200807. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pancholi P., Mirza A., Bhardwaj N., Steinman R. M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993 May 14;260(5110):984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- Philips M. R., Buyon J. P., Winchester R., Weissmann G., Abramson S. B. Up-regulation of the iC3b receptor (CR3) is neither necessary nor sufficient to promote neutrophil aggregation. J Clin Invest. 1988 Aug;82(2):495–501. doi: 10.1172/JCI113623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Jackson B. K., Beachey E. H., Kang A. H. Formation of multinucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen-and mitogen-stimulated lymphocytes. J Exp Med. 1982 Jan 1;155(1):168–178. doi: 10.1084/jem.155.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzis S., Chatterjee D., Brennan P. J. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J Gen Microbiol. 1993 Nov;139(11):2649–2658. doi: 10.1099/00221287-139-11-2649. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Reichhold N., Ryan G. B. Degradation of granuloma-inducing micro-organisms by macrophages. J Pathol. 1970 Aug;101(4):339–354. doi: 10.1002/path.1711010407. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Sullivan L., Sano S., Pirmez C., Salgame P., Mueller C., Hofman F., Uyemura K., Rea T. H., Bloom B. R., Modlin R. L. Expression of adhesion molecules in leprosy lesions. Infect Immun. 1991 Nov;59(11):4154–4160. doi: 10.1128/iai.59.11.4154-4160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey E., Zhang J. H., Dayer J. M. IFN-gamma and 1,25(OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol. 1992 Sep 15;149(6):2040–2046. [PubMed] [Google Scholar]
- Voraberger G., Schäfer R., Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5'-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991 Oct 15;147(8):2777–2786. [PubMed] [Google Scholar]
- Zhang Y., Doerfler M., Lee T. C., Guillemin B., Rom W. N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993 May;91(5):2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Rom W. N. Regulation of the interleukin-1 beta (IL-1 beta) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol. 1993 Jun;13(6):3831–3837. doi: 10.1128/mcb.13.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]