Abstract
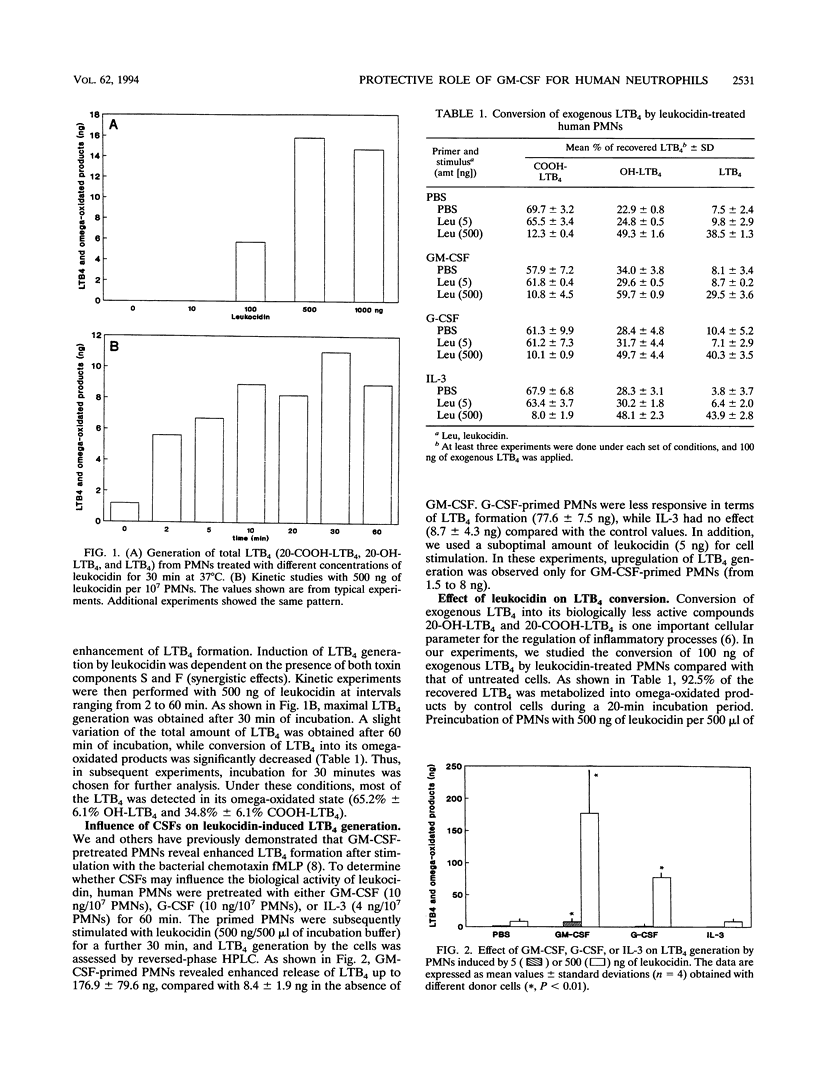
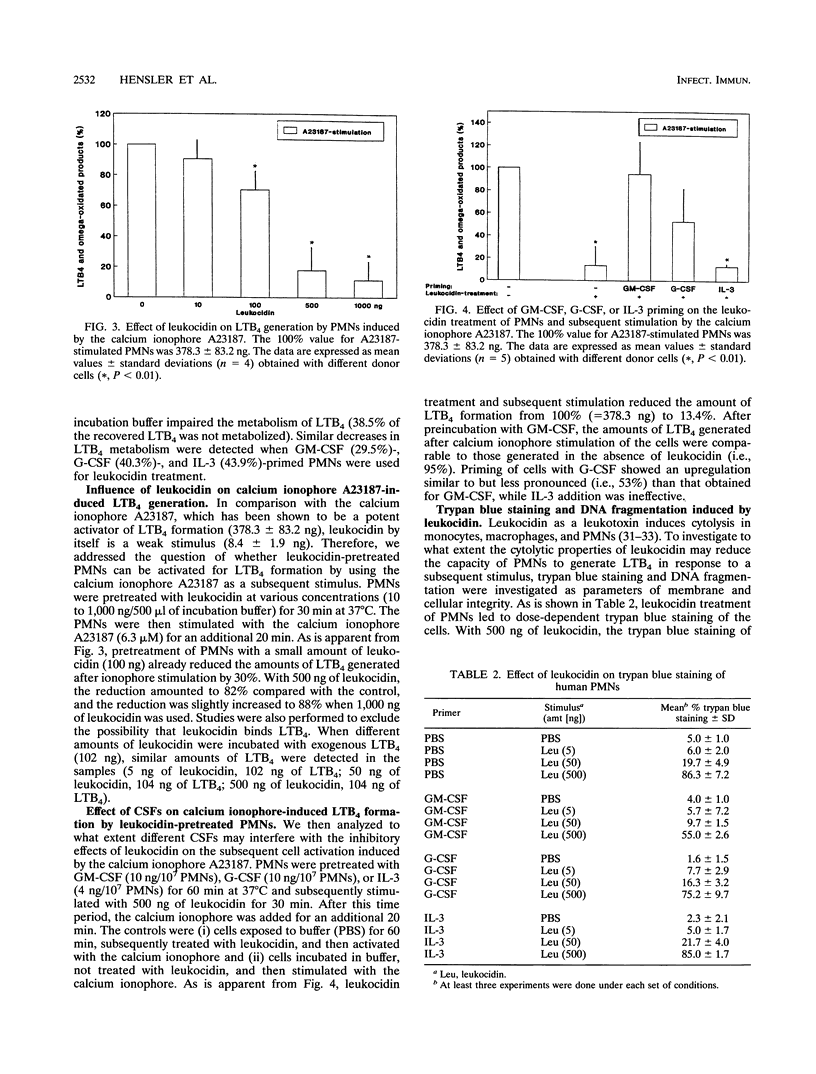
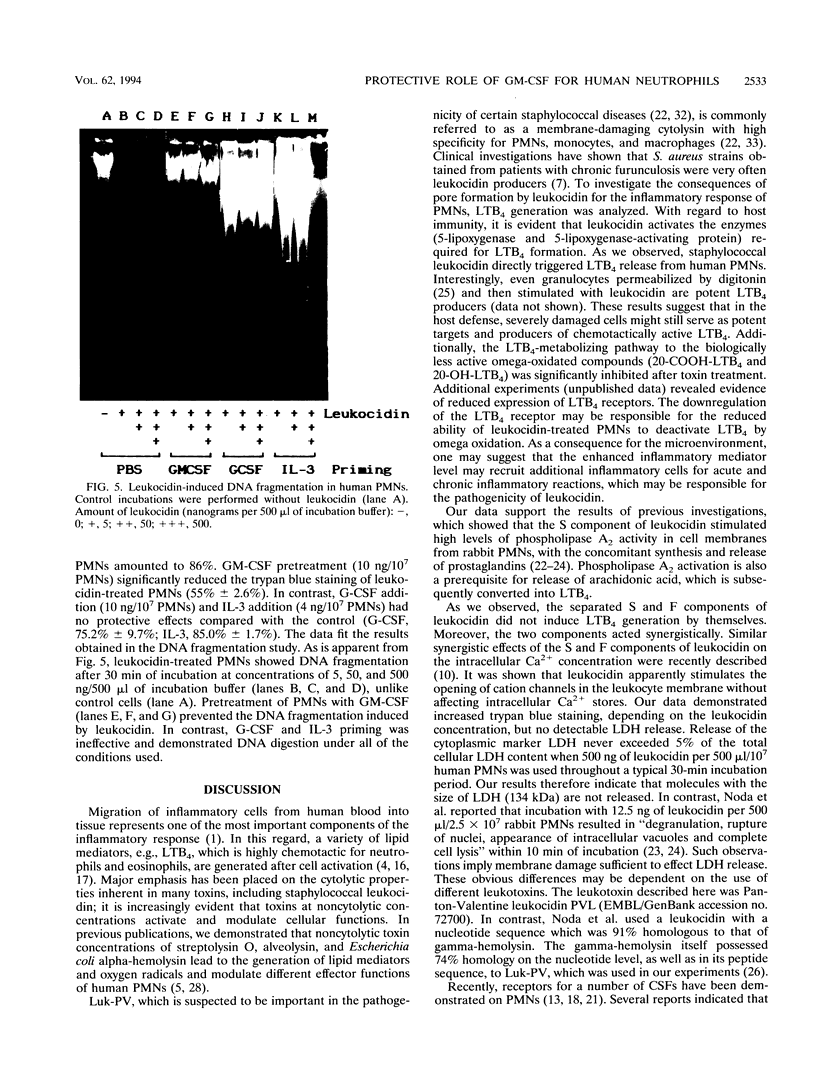
We studied the effect of leukocidin from Staphylococcus aureus V8 strains (Luk-PV) on the generation of Leukotriene B4 (LTB4) and its metabolites from human polymorphonuclear neutrophils (PMNs). Significant amounts of LTB4 were generated by PMNs after leukocidin exposure in a time- and dose-dependent manner, as shown by reversed-phase high-performance liquid chromatography analysis. In this regard, the S and F components of leukocidin acted synergistically. The calcium ionophore A23187 induced LTB4 generation, and the metabolism of exogenously added LTB4 into biologically less active omega-oxidated compounds was significantly decreased after leukocidin exposure. Priming of PMNs with granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF prior to leukocidin exposure substantially increased toxin- and calcium ionophore A23187-induced LTB4 formation. The inhibitory effects of leukocidin on mediator release were accompanied by membrane damage and DNA fragmentation, which were both restored after pretreatment with GM-CSF. The data suggest that the presence of costimulatory priming factors such as GM-CSF or G-CSF in the microenvironment of an inflammatory focus determines the pathophysiological effects induced by S. aureus leukocidin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff S. C., de Weck A. L., Dahinden C. A. Interleukin 3 and granulocyte/macrophage-colony-stimulating factor render human basophils responsive to low concentrations of complement component C3a. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6813–6817. doi: 10.1073/pnas.87.17.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. A. Leukotrienes in inflammation. Agents Actions. 1986 Oct;19(1-2):87–99. doi: 10.1007/BF01977263. [DOI] [PubMed] [Google Scholar]
- Bremm K. D., König W., Pfeiffer P., Rauschen I., Theobald K., Thelestam M., Alouf J. E. Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun. 1985 Dec;50(3):844–851. doi: 10.1128/iai.50.3.844-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom J., Schönfeld W., König W. Metabolism of leukotriene B4 by activated human polymorphonuclear granulocytes. Immunology. 1988 Jul;64(3):509–518. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cribier B., Prévost G., Couppie P., Finck-Barbançon V., Grosshans E., Piémont Y. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology. 1992;185(3):175–180. doi: 10.1159/000247443. [DOI] [PubMed] [Google Scholar]
- Dahinden C. A., Zingg J., Maly F. E., de Weck A. L. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988 Apr 1;167(4):1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Finck-Barbançon V., Prévost G., Piémont Y. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res Microbiol. 1991 Jan;142(1):75–85. doi: 10.1016/0923-2508(91)90099-v. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., MUDD S., HOCHSTEIN H. D., LENHART N. A. The assay of anti-staphylococcal leucocidal components (F and S) in human serum. Br J Exp Pathol. 1962 Jun;43:295–312. [PMC free article] [PubMed] [Google Scholar]
- Hansson G., Lindgren J. A., Dahlén S. E., Hedqvist P., Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 Jul 20;130(1):107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Structure-function relationships of the hematopoietic growth factors. Proteins. 1992 Jan;12(1):1–9. doi: 10.1002/prot.340120102. [DOI] [PubMed] [Google Scholar]
- Köller M., Hensler T., König B., Prévost G., Alouf J., König W. Induction of heat-shock proteins by bacterial toxins, lipid mediators and cytokines in human leukocytes. Zentralbl Bakteriol. 1993 Apr;278(2-3):365–376. doi: 10.1016/s0934-8840(11)80853-4. [DOI] [PubMed] [Google Scholar]
- Köller M., König W., Brom J., Erbs G., Müller F. E. Studies on the mechanisms of granulocyte dysfunctions in severely burned patients--evidence for altered leukotriene generation. J Trauma. 1989 Apr;29(4):435–445. doi: 10.1097/00005373-198904000-00004. [DOI] [PubMed] [Google Scholar]
- König W., Schönfeld W., Raulf M., Köller M., Knöller J., Scheffer J., Brom J. The neutrophil and leukotrienes--role in health and disease. Eicosanoids. 1990;3(1):1–22. [PubMed] [Google Scholar]
- Lloyd A. R., Oppenheim J. J. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992 May;13(5):169–172. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. P., McColl S. R., Naccache P. H., Borgeat P. Activation of the human neutrophil 5-lipoxygenase by leukotriene B4. Br J Pharmacol. 1992 Sep;107(1):226–232. doi: 10.1111/j.1476-5381.1992.tb14491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Noda M., Kato I., Hirayama T., Matsuda F. Fixation and inactivation of staphylococcal leukocidin by phosphatidylcholine and ganglioside GM1 in rabbit polymorphonuclear leukocytes. Infect Immun. 1980 Aug;29(2):678–684. doi: 10.1128/iai.29.2.678-684.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Kato I., Hirayama T., Matsuda F. Mode of action of staphylococcal leukocidin: effects of the S and F components on the activities of membrane-associated enzymes of rabbit polymorphonuclear leukocytes. Infect Immun. 1982 Jan;35(1):38–45. doi: 10.1128/iai.35.1.38-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Waldron P. E., Friend D., Sassenfeld H. M., Price V., Anderson D., Cosman D., Andrews R. G., Bernstein I. D., Urdal D. L. Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood. 1989 Jul;74(1):56–65. [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Scheffer J., König W., Braun V., Goebel W. Comparison of four hemolysin-producing organisms (Escherichia coli, Serratia marcescens, Aeromonas hydrophila, and Listeria monocytogenes) for release of inflammatory mediators from various cells. J Clin Microbiol. 1988 Mar;26(3):544–551. doi: 10.1128/jcm.26.3.544-551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem J. 1960 Apr;75:158–165. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The participation of phospholipids in the interaction of leucocidin and the cell membrane of the polymorphonuclear leucocyte. Biochem J. 1967 Dec;105(3):1029–1038. doi: 10.1042/bj1051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiaga A. M., Munoz E., Huber B. T. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J Immunol. 1992 Jul 1;149(1):107–112. [PubMed] [Google Scholar]