Abstract
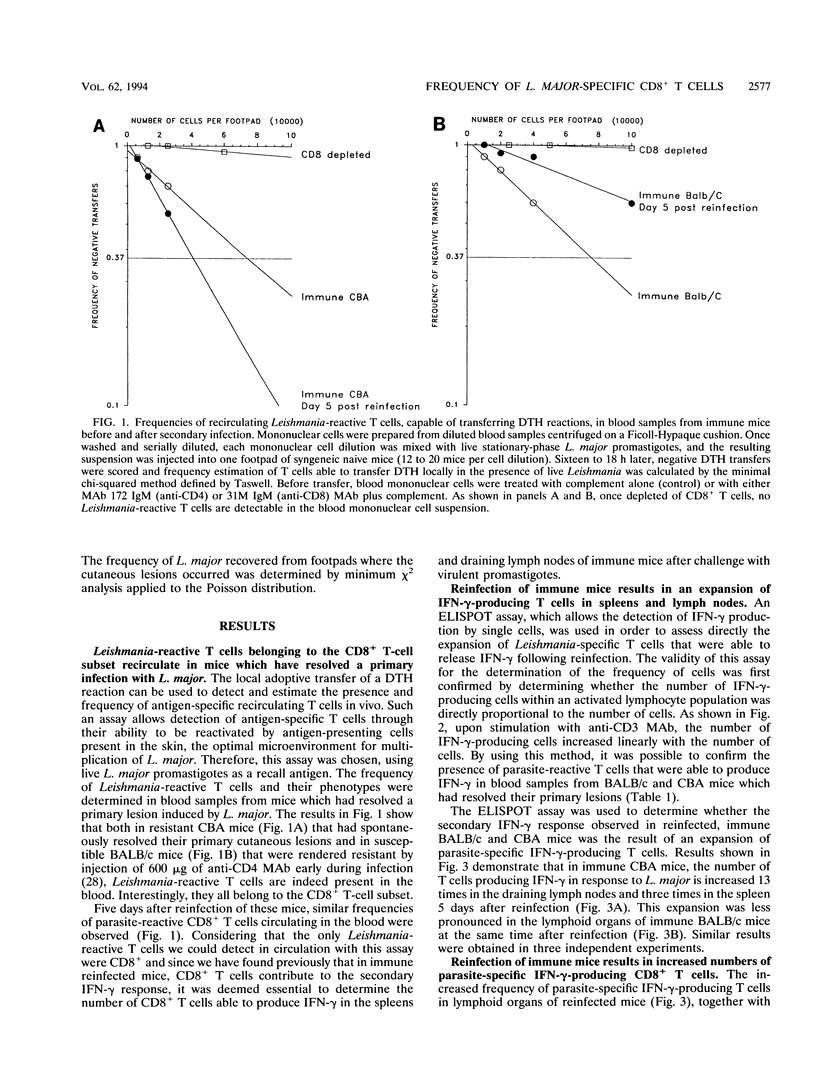
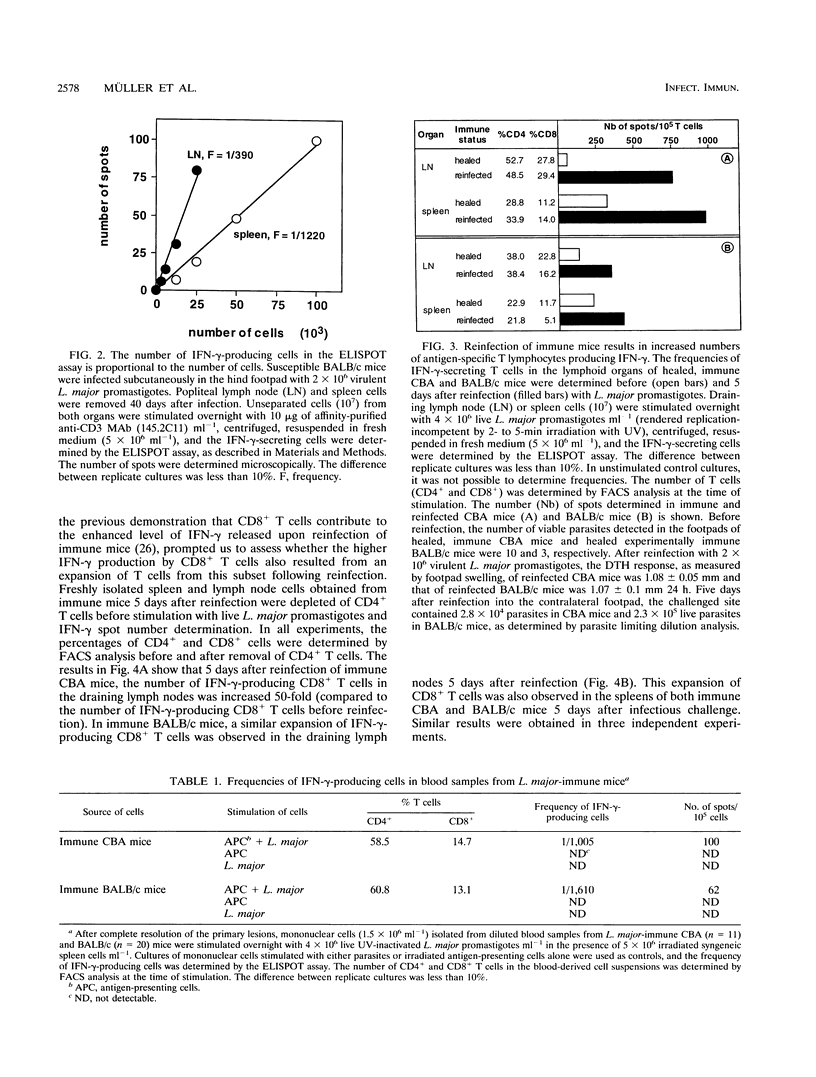
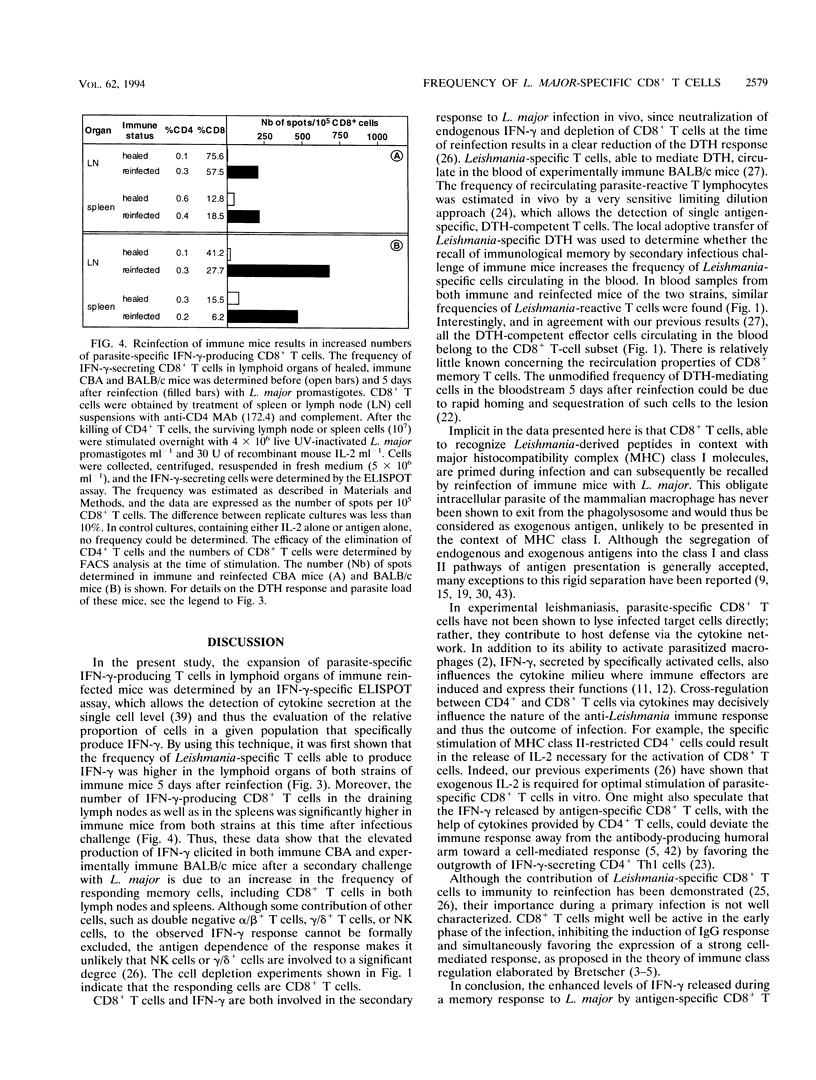
Reinfection of immune mice with Leishmania major elicits a secondary gamma interferon (IFN-gamma) response to which specific CD8+ T cells are essential. We have shown previously that specific CD8+ T cells from reinfected immune mice release substantially higher levels of IFN-gamma, a cytokine essential for the efficient activation of parasitized macrophages to kill intracellular L. major. By using an ELISPOT assay, which allows the detection of IFN-gamma production by individual cells, it is shown here that this elevated IFN-gamma response is the result of an increase of up to 50-fold in the frequency of parasite-specific CD8+ T lymphocytes in the spleens and draining lymph nodes of both immune reinfected CBA and BALB/c mice. This observation is additional evidence of the role that CD8+ T cells play in immunity to reinfection with L. major.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behin R., Mauel J., Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979 Aug;48(1):81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Bretscher P. A. On the control between cell-mediated, IgM and IgG immunity. Cell Immunol. 1974 Aug;13(2):171–195. doi: 10.1016/0008-8749(74)90237-8. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A. Significance and mechanisms of cellular regulation of the immune response. Fed Proc. 1981 Apr;40(5):1473–1478. [PubMed] [Google Scholar]
- Bretscher P. A. The regulatory functions of CD4+ and CD8+ T-cell subsets in immune class regulation. Res Immunol. 1991 Jan;142(1):45–50. doi: 10.1016/0923-2494(91)90011-7. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Chen B. D., Clark C. R. Interleukin 3 (IL 3) regulates the in vitro proliferation of both blood monocytes and peritoneal exudate macrophages: synergism between a macrophage lineage-specific colony-stimulating factor (CSF-1) and IL 3. J Immunol. 1986 Jul 15;137(2):563–570. [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Denkers E. Y., Gazzinelli R. T., Hieny S., Caspar P., Sher A. Bone marrow macrophages process exogenous Toxoplasma gondii polypeptides for recognition by parasite-specific cytolytic T lymphocytes. J Immunol. 1993 Jan 15;150(2):517–526. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Clark K., Benacerraf B., Rock K. L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Loss G. E., Jr, Elias C. G., Fields P. E., Ribaudo R. K., McKisic M., Sant A. J. Major histocompatibility complex class II-restricted presentation of an internally synthesized antigen displays cell-type variability and segregates from the exogenous class II and endogenous class I presentation pathways. J Exp Med. 1993 Jul 1;178(1):73–85. doi: 10.1084/jem.178.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J., Moedder E., Behin R., Engers H. Recognition of protozoan parasite antigens by murine T lymphocytes. I. Induction of specific T lymphocyte-dependent proliferative response to Leishmania tropica. Eur J Immunol. 1979 Nov;9(11):841–847. doi: 10.1002/eji.1830091103. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Sordat B., Zaech P., Maryanski J. L., Bron C. Age-associated increase in expression of the T cell surface markers Thy-1, Lyt-1, and Lyt-2 in congenitally athymic (nu/nu) mice: analysis by flow microfluorometry. J Immunol. 1981 Mar;126(3):865–870. [PubMed] [Google Scholar]
- Mackay C. R. Immunological memory. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- McMenamin C., Holt P. G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993 Sep 1;178(3):889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milon G., Titus R. G., Cerottini J. C., Marchal G., Louis J. A. Higher frequency of Leishmania major-specific L3T4+ T cells in susceptible BALB/c as compared with resistant CBA mice. J Immunol. 1986 Feb 15;136(4):1467–1471. [PubMed] [Google Scholar]
- Murray H. W., Squires K. E., Miralles C. D., Stoeckle M. Y., Granger A. M., Granelli-Piperno A., Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992 Mar 15;148(6):1858–1863. [PubMed] [Google Scholar]
- Müller I., Kropf P., Etges R. J., Louis J. A. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect Immun. 1993 Sep;61(9):3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Kropf P., Louis J., Milon G. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite-specific CD8+ T cells. Int Immunol. 1991 Jun;3(6):587–597. doi: 10.1093/intimm/3.6.587. [DOI] [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Louis J. A. Experimentally induced cutaneous leishmaniasis: are L3T4+ T cells that promote parasite growth distinct from those mediating resistance? Immunol Lett. 1988 Nov;19(3):251–259. doi: 10.1016/0165-2478(88)90151-4. [DOI] [PubMed] [Google Scholar]
- Müller I. Role of T cell subsets during the recall of immunologic memory to Leishmania major. Eur J Immunol. 1992 Dec;22(12):3063–3069. doi: 10.1002/eji.1830221206. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Prat M., Gribaudo G., Comoglio P. M., Cavallo G., Landolfo S. Monoclonal antibodies against murine gamma interferon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires K. E., Schreiber R. D., McElrath M. J., Rubin B. Y., Anderson S. L., Murray H. W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989 Dec 15;143(12):4244–4249. [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990 Jul 1;145(1):68–77. [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Detection of individual mouse splenic T cells producing IFN-gamma and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990 Mar 27;128(1):65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Kelso A., Louis J. A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Tuttosi S., Bretscher P. A. Antigen-specific CD8+ T cells switch the immune response induced by antigen from an IgG to a cell-mediated mode. J Immunol. 1992 Jan 15;148(2):397–403. [PubMed] [Google Scholar]
- Weiss S., Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991 Feb 22;64(4):767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]



