Abstract
Susceptibility of mice to infection with Yersinia enterocolitica has been shown to be related to neither the Ity locus encoding for resistance to Salmonella typhimurium and other pathogens nor the H-2 locus. Recent studies in our laboratory have demonstrated that T-cell-mediated immune responses are required for overcoming primary Yersinia infection. In the present study, we investigated the course of infection with Y. enterocolitica and the resulting immune responses in Yersinia-susceptible BALB/c and Yersinia-resistant C57BL/6 mice. In the early phase of infection, the clearance of the pathogen was comparable in both strains of mice, suggesting similar mechanisms of innate resistance. Splenic T cells from Yersinia-infected C57BL/6 mice exhibited marked proliferative responses and produced gamma interferon (IFN-gamma) upon exposure to heat-killed yersiniae. By contrast, the Yersinia-specific T-cell response in BALB/c mice was weak, and IFN-gamma production could not be detected before day 21 postinfection. T cells isolated from C57BL/6 mice 7 days after infection mediated immunity to Y. enterocolitica but those from BALB/c mice did not, while at 21 days postinfection T cells from both strains mediated protection. Neutralization of IFN-gamma abrogated resistance to yersiniae in C57BL/6 mice but to a far smaller extent in BALB/c mice. Administration of recombinant IFN-gamma or anti-interleukin-4 antibodies rendered BALB/c mice resistant to yersiniae, whereas this treatment did not significantly affect the course of the infection in C57BL/6 mice. These results indicate that the cellular immune response, in particular the production of IFN-gamma by Yersinia-specific T cells, is associated with resistance of mice to Y. enterocolitica.
Full text
PDF


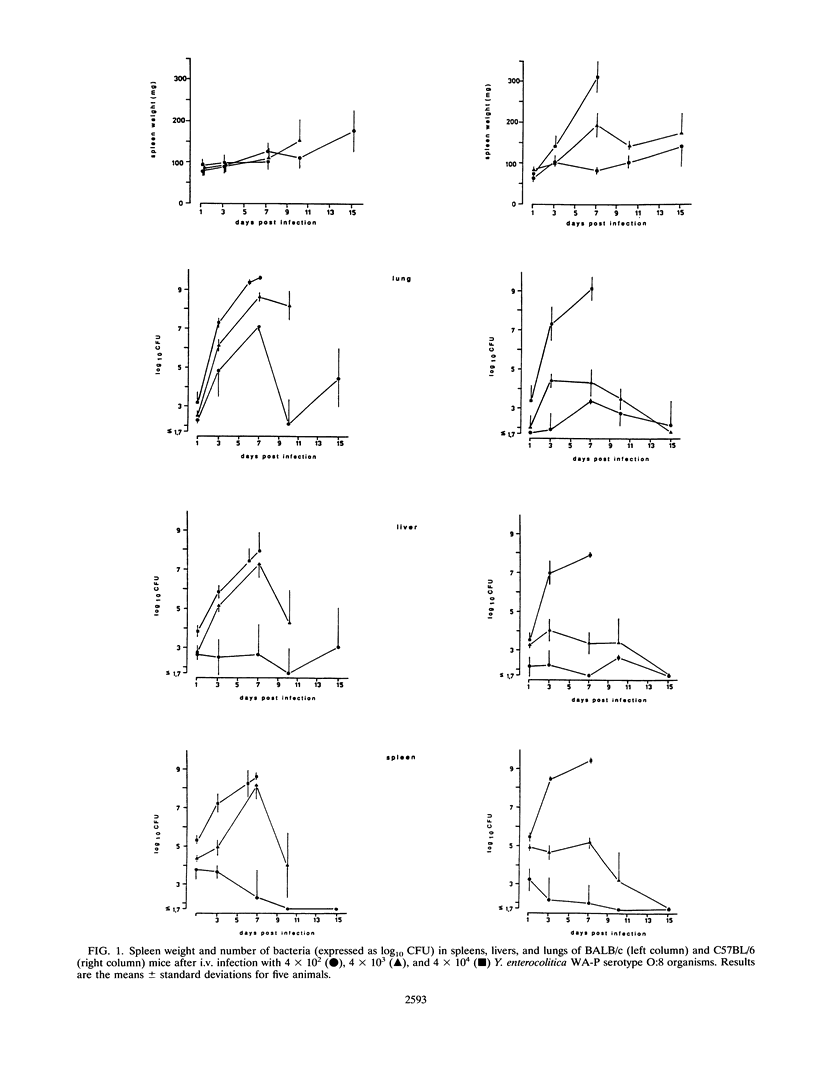
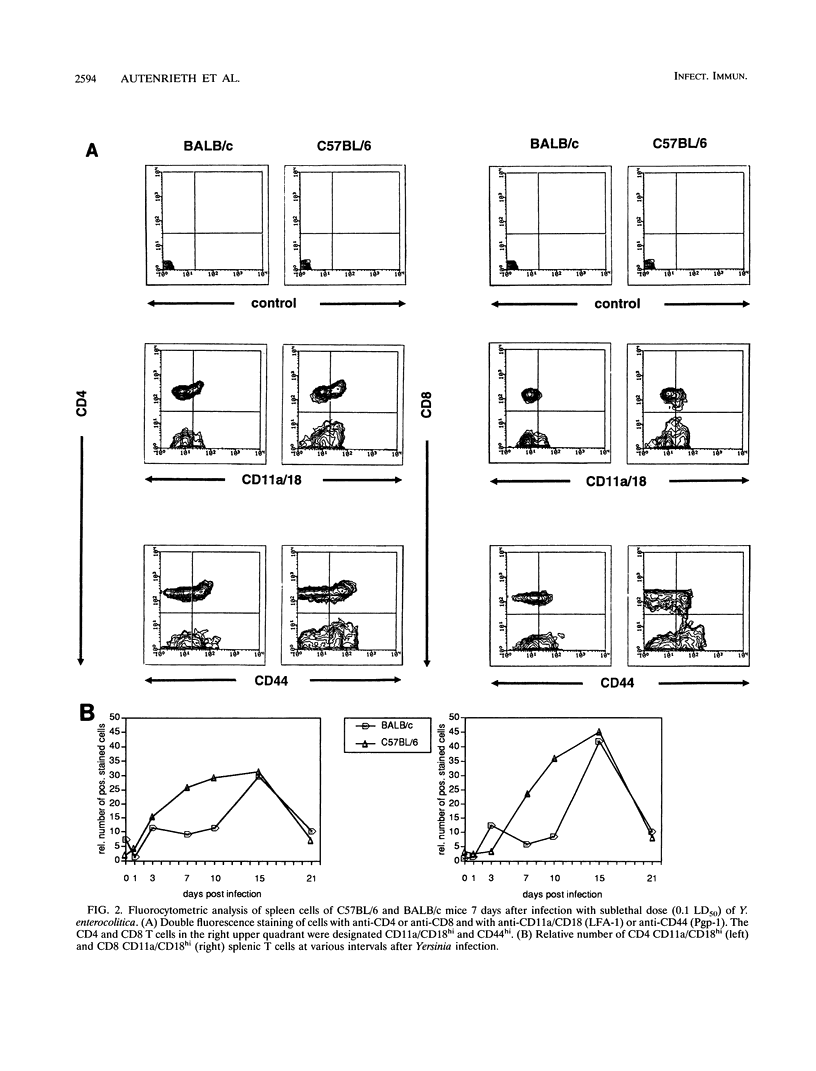
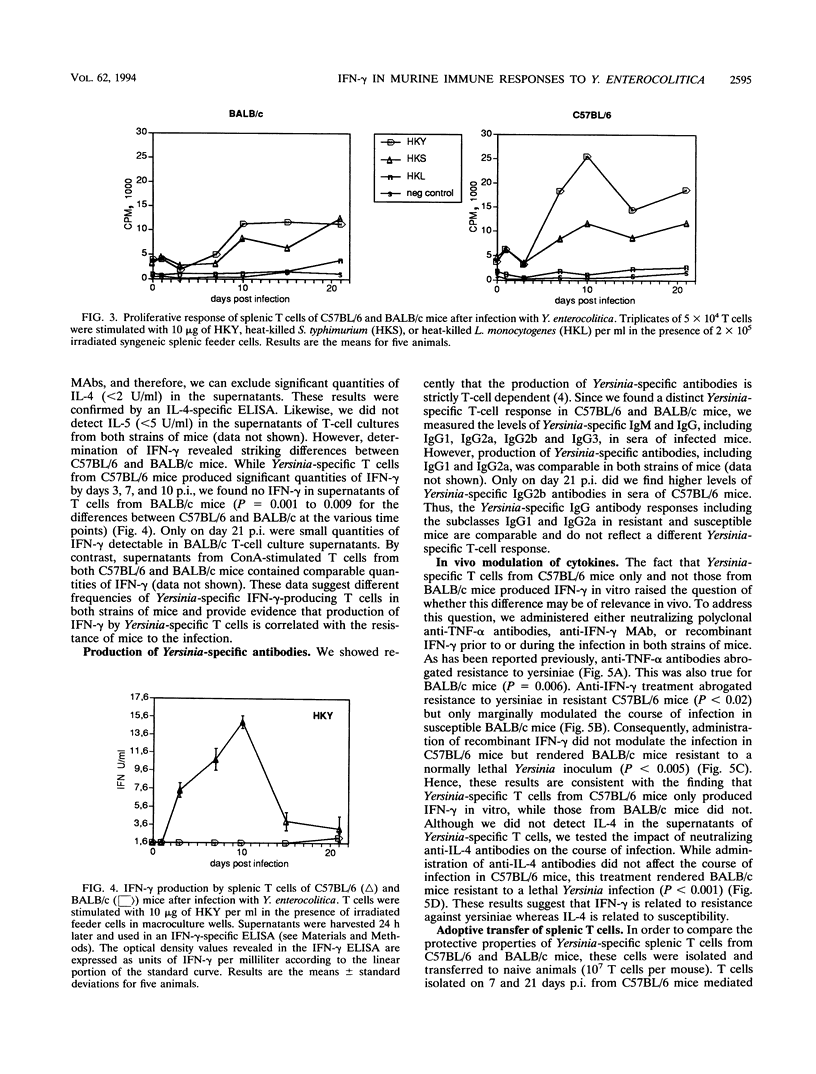
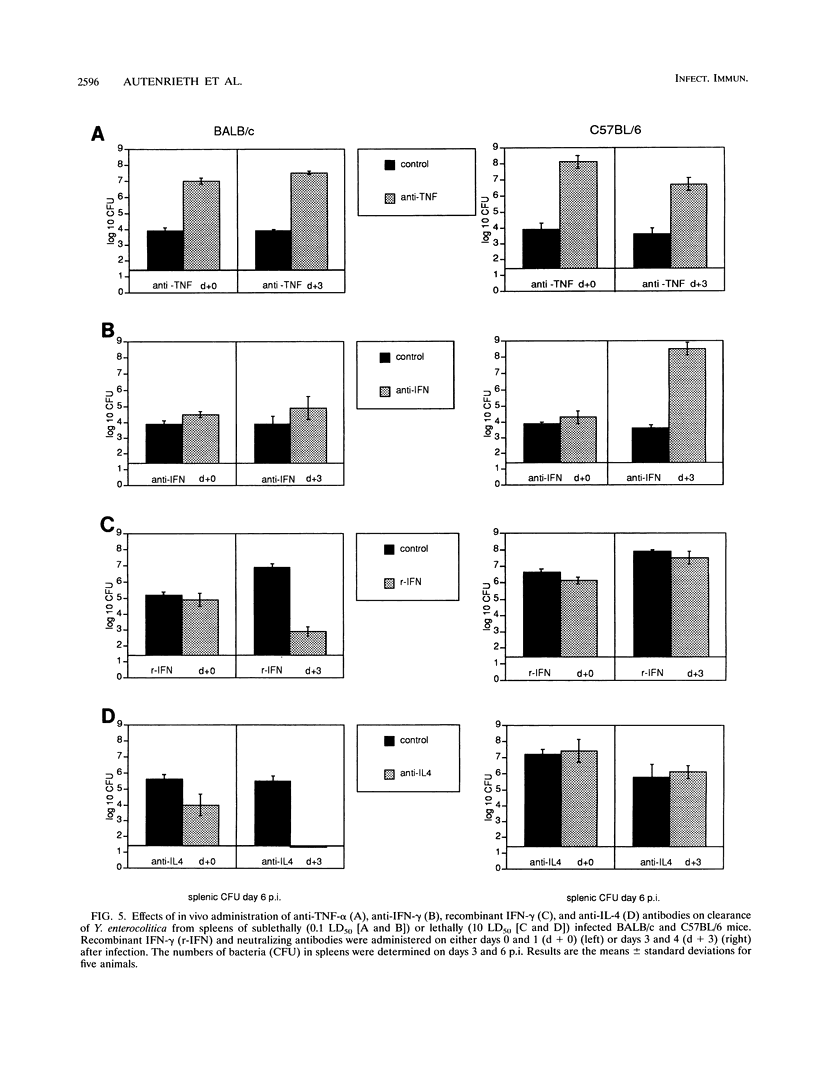



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autenrieth I. B., Hantschmann P., Heymer B., Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993 Jan;187(1-2):1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1992;181(6):333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Tingle A., Reske-Kunz A., Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect Immun. 1992 Mar;60(3):1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth I. B., Vogel U., Preger S., Heymer B., Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993 Jun;61(6):2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Gessner A., Röllinghoff M. Cytokines in leishmaniasis: a complex network of stimulatory and inhibitory interactions. Immunobiology. 1993 Nov;189(3-4):356–396. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A., Wei G., Menon J. N., Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992 Jul 24;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Aber R. C. Yersinia enterocolitica. N Engl J Med. 1989 Jul 6;321(1):16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- Haak-Frendscho M., Brown J. F., Iizawa Y., Wagner R. D., Czuprynski C. J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992 Jun 15;148(12):3978–3985. [PubMed] [Google Scholar]
- Hancock G. E., Schaedler R. W., MacDonald T. T. Multigenic control of resistance to Yersinia enterocolitica in inbred strains of mice. Infect Immun. 1988 Feb;56(2):532–533. doi: 10.1128/iai.56.2.532-533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock G. E., Schaedler R. W., MacDonald T. T. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun. 1986 Jul;53(1):26–31. doi: 10.1128/iai.53.1.26-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski C., Kutschka U., Schmoranzer H. P., Naumann M., Stallmach A., Hahn H., Menge H., Riecken E. O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989 Mar;57(3):673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg K., Reimann J., Kabelitz D., Hardt C., Wagner H. A rapid colorimetric assay for the determination of IL-2-producing helper T cell frequencies. J Immunol Methods. 1985 Mar 18;77(2):237–246. doi: 10.1016/0022-1759(85)90036-5. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Gaede K., Autenrieth I. B. Experimental Yersinia enterocolitica infection in rodents: a model for human yersiniosis. APMIS. 1993 Jun;101(6):417–429. [PubMed] [Google Scholar]
- Heesemann J., Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983 Aug;155(2):761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Curry A. J., Blackwell J. M. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991 Apr 15;146(8):2763–2770. [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kono D. H., Ogasawara M., Yu D. T. An animal model to study serum immunoglobulin response to infection by Yersinia enterocolitica. Clin Exp Rheumatol. 1985 Apr-Jun;3(2):117–121. [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Louis J. A. Immunology of leishmaniasis. Curr Opin Immunol. 1992 Aug;4(4):413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Müller I., Fruth U., Louis J. A. Immunobiology of experimental leishmaniasis. Med Microbiol Immunol. 1992;181(1):1–12. doi: 10.1007/BF00193391. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Powrie F., Coffman R. L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993 Jun;14(6):270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Locksley R. M. The worm and the protozoa: stereotyped responses or distinct antigens? Parasitol Today. 1993 Jul;9(7):258–260. doi: 10.1016/0169-4758(93)90071-m. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Nussenzweig R. S., Romero P., Zavala F. The in vivo cytotoxic activity of CD8+ T cell clones correlates with their levels of expression of adhesion molecules. J Exp Med. 1992 Apr 1;175(4):895–905. doi: 10.1084/jem.175.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Richard S., Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990 Mar;58(3):841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel U., Autenrieth I. B., Berner R., Heesemann J. Role of plasmid-encoded antigens of Yersinia enterocolitica in humoral immunity against secondary Y. enterocolitica infection in mice. Microb Pathog. 1993 Jul;15(1):23–36. doi: 10.1006/mpat.1993.1054. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]



