Abstract
Background
While tissue engineering offers promise for organ and tissue transplantation, it can also be used to examine transplant and immune biology. Endothelial cells engrafted within three-dimensional matrices create stable units that produce all of the factors of a functional quiescent endothelium. Perivascular implantation of tissue engineered endothelial cell constructs provides long-term control of vascular repair after injury. This control is established without restoration of the natural luminal:mural endothelium, and most intriguingly, without engendering host allo- and xenogeneic immune responses. We examined how endothelial immunogenicity is controlled by interaction with three-dimensional matrices.
Materials and Methods
Human aortic endothelial cells (HAE) were either grown to confluence on polystyrene tissue culture plates or within three-dimensional collagen-based matrices. MHC II, integrin, interferon (IFN)-γ receptor expression and signaling were analyzed via confocal microscopy, flow cytometry, RT-PCR, and microarray. Splenocyte proliferation was assayed by thymidine incorporation.
Results
Despite similar expression levels of IFN-γ receptors, matrix-embedded HAE elicited far less STAT-1-phosphorylation upon IFN-γ stimulation, and expressed 2-fold less MHC II, than HAE grown to confluence on culture plates (p<0.001). This effect correlated with reduced expression of integrin αv and β3 (p<0.002), and muted proliferation of porcine splenocytes (p<0.001).
Conclusion
Matrix architecture is critical for modulation of endothelial immunogenicity. Embedding HAE within a physiologic three-dimensional environment affects activity of intracellular signaling pathways, MHC II expression, and subsequent activation of immune cells. These findings might offer novel insights to our understanding of endothelial-mediated diseases and might enhance our ability to leverage the potential for cell-based therapies.
INTRODUCTION
Tissue engineering and regenerative medicine use principles of cell transplantation, material science, and bioengineering to construct biological substitutes for restoration of normal function in diseased and injured tissues.1, 2 Such cell-based therapies make use of the full spectrum of physiologically responsive control mechanisms of intact cells. Tissue engineered vascular and cardiovascular components, bones, cartilages and gastrointestinal tissues have all been produced for therapy and to study function-structure relationships.3–6 Indeed these cellular constructs can be implanted in virtually any given space and at any location. We successfully used perivascular implants of tissue engineered endothelial cell constructs to demonstrate that endothelial cells do not need to reside at the intimal vessel lumen to exert regulatory functionality.7–10 Endothelial cell function is density dependent.11,12 Confluent endothelial cells promote vascular quiescence and subconfluent or sparsely populated cells induce growth and the healing-associated phenomena that include stimuli for auto- and paracrine hyperplasia, enhanced vasomotor tone and even local vasoconstriction. Embedding endothelial cells within three-dimensional collagen based matrices (Gelfoam) allows these cells to attain confluence in a controlled environment. Such constructs enable endothelial cells to retain the status of endothelial quiescence, secretion of essential regulatory factors, and the associated potential for vasoregulatory control. These vehicles can be stored, manipulated, functionally validated and implanted at will at sites protected from environmental forces, e.g. in the immediate vicinity of a diseased vessel.7, 8, 10, 13
Equally fascinating is the similar efficacy of allo- and xenogeneic endothelial cell implants.8, 10 Three dimensional matrix-embedded endothelial cell constructs evidence reduced expression levels of cytokine-inducible adhesion, costimulatory and MHC class II molecules compared with cell suspensions of endothelial cells or endothelial cells grown to confluence on polystyrene tissue culture plates.14 As the level of MHC II expression has been directly linked with endothelial immunogenicity15 we now compare IFN-γ receptor expression, STAT1-phosphorylation and MHC II expression between matrix-embedded endothelial cells and endothelial cells grown on tissue culture plates. Such immune modulation is correlated with endothelial integrin expression patterns and with xenogeneic splenocyte proliferation.
METHODS
Human aortic endothelial cells
Primary human aortic EC (HAE, Cambrex, MD) were grown in optimized endothelial growth medium-2 (EGM-2, Cambrex, MD) supplemented with 5% fetal bovine serum (FBS, Life Technologies, NY). Confluent monolayers of second to fifth passage were used for the experiments. Gelfoam sheets (Pfizer, NY) were cut into 2.5x1.0x0.3 cm3 blocks, hydrated in PBS for 30 min and then incubated with 0.9 x105 HAE for 2 hours before transfer to 17x100-mm polypropylene tubes containing 3 ml complete EGM-2 medium as previously described.14 HAE were stimulated with 1000 U IFN-γ/ml (Roche) for indicated time periods at 37°C in a humidified air atmosphere containing 5% CO2.
RT-PCR
Total RNA was extracted from HAE grown on TCPS or matrix-embedded after stimulation with IFN-γ for indicated time periods using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Complementary DNA was synthesized using the TaqMan reverse transcription reagents from Applied Biosystems (Foster City, CA). Real-time PCR analysis was performed with an Opticon Real Time PCR Machine (MJ Research) using SYBR Green PCR Master Mix Reagent Kit (Applied Biosystems). Data from the reaction were collected and analyzed by the complementary Opticon computer software (MJ Research). Gene expression was quantified from standard curves and normalized to GAPDH.
Microarray Analysis
Integrin expression was compared between HAEC grown to confluence on polystyrene tissue culture plates and embedded within Gelfoam matrices using the extracellular matrix and adhesion molecule microarray assay (SuperArray Biosciences, Frederick, MD, USA). Final hybridization was performed with CDP-Star chemiluminescent substrate followed by exposure and analysis on a FluorChem SP (Alpha Innotech, San Leandro, CA).
Confocal Microscopy
For immunofluorescence analysis, HAE were washed in PBS and fixed with 3.7% paraformaldehyde for 15 min at room temperature. After blocking for 60 min in blocking buffer (PBS/10%FCS/0.1% sodium azide) cells were twice washed in washing buffer (PBS/0.05%glycine/0.75%BSA). Immunostainings were performed by incubating cells with primary antibodies overnight at 4°C diluted in blocking buffer: mouse-anti human IFN-γ receptor I (USBiological, Swampscott, MA; 1:100) or mouse-anti human IFN-γ receptor II (USBiological, 25 mg/ml). After three washes with washing buffer detection of bound primary antibody was visualized by the addition of specific Alexa Fluor 488-conjugated secondary antibodies (Invitrogen) for one hour at room temperature (7.5 mg/ml). Images were collected with a Zeiss LSM510 confocal laser-scanning microscope (Thornwood, NY).
Flow-cytometry
EC monolayers or EC embedded in Gelfoam were harvested after stimulation with IFN-γ for indicated time periods. Media were aspirated and cells were washed with PBS. Monolayers were incubated in 1.0 mM PBS/EDTA for 5 min, and disrupted by gentle shaking. Gelfoam were digested with collagenase type I, shown to have no effect on IFN-γ receptor (IFN-γR) and HLA-DR-expression. Cell-suspensions were washed and 3x105 cells were resuspended in FACS buffer (PBS containing 0,1% BSA and 0,1% sodium azide, Sigma Chemicals; St. Louis, MO). Endothelial cells were incubated with mouse anti-human HLA-DP,DQ,DR (clone CR3/43, fluorescein-conjugated; DakoCytomation, Carpinteria, CA), anti-human IFN-γR chain 1 (clone MMHGR-1) or a polyclonal rabbit anti-human IFN-γR chain 2 (both from Fitzgerald Industries, Concord MA) for 30 min at 4°C. Cells were washed and if necessary stained with a secondary antibody (fluorescein-conjugated rat anti-mouse IgG1, clone A85-1; BD Pharmingen, San Diego, CA or goat anti-rabbit IgG, Fitzgerald Industries) for 30 min at 4°C. Cells were then washed, fixed in 1% paraformaldehyde, and 105 cells were analyzed by flow cytometry using a FACScalibur instrument and CellQuest software (Becton Dickinson, San Diego, CA).
Western blot analysis
Cells isolated from Gelfoam matrices by collagenase-treatment were washed in PBS buffer, and cell lysates were prepared by incubation with lysis buffer (20 mM Tris, 150 mM NaCl, pH 7.5, 1% Triton X-100, 1% deoxycholate, 0.1% SDS and protease inhibitor; Roche). Total protein concentration within cell lysates was determined using a bicinochonic acid assay kit (Pierce, Rockford, IL). Samples were separated on 4–20% Ready Tris-HCl gels (BioRad Laboratories, Hercules, CA). As positive control for signal transducer and activator of transcription (STAT)-1 3T3-L1 cells +LIF (Biosource, Camarillo, CA) were used. Proteins were then transferred onto PVDF membranes (Millipore, Billerica, MA) by using glycin-Tris transfer buffer. Blot membranes were blocked in Starting Block blocking buffer (Pierce, Rockford, IL) for 1 hour. For detection of STAT-1 (rabbit anti-human STAT1 and rabbit anti-human STAT1 [pY701] both from Biosource) blocked membranes were incubated with the respective antibodies at a dilution of 1:200 in blocking buffer overnight at 4°C. Membranes were then washed three times at room temperature with wash buffer consisting of PBS with 0.05% Tween 20, and then incubated with secondary antibodies conjugated to horseradish peroxidase (donkey anti-rabbit IgG1; Promega, Madison, WI) at a 1:3.000 dilution in blocking buffer for 2 h at room temperature. This was followed by washing in five changes of wash buffer. For detection of specific protein bands, the blot was incubated with chemiluminescence substrate from the Western Lightning Chemiluminescence Reagent Plus kit (Perkin-Elmer, Boston, MA) according to the manufacturer’s instructions. This step was followed by exposure to X-ray film (Kodak X-Omat Blue XB-1).
Lymphocyte proliferation assay
HAE grown on polystyrene wells or embedded in Gelfoam were seeded in 96 well plates at 5x104 cells/well and stimulated with 1000 U/ml IFN-γ for 48 hours, followed by mitomycin C treatment (Sigma, 50 μg/ml for 30 min) to prevent background proliferation. Spleens harvested from LargeWhite adult swine were cut in several pieces under sterile conditions. Clumps were immersed in solution and further dispersed by drawing and expelling the suspension several times through a sterile syringe with a 19-G needle. The suspension was filtered through a 200 μm mesh nylon screen to remove debris. Erythrocytes were lysed by treatment with ACK buffer (Cambrex, Walkersville, MD) for 5 minutes at room temperature. Remaining cells were washed twice with RPMI (containing 2 mM L-glutamine, 0.1 M HEPES, 200 U/ml Penicillin G, 200 μg/ml streptomycin, 5% heat-inactivated calf serum, Life Technologies) and added at 2x105 cells/well. In some experiments a murine antibody directed against HLA-DP,DQ,DR blocked activation via MHC class II molecules. 3[H]-thymidine incorporation was measured on day 6 by 16h pulse (1 μCi/ml, Amersham). Thymidine uptake of mitomycin-treated PAE, medium or T-cells alone was used as negative controls.
Statistics
Statistical analyses were performed with JMP software (SAS Institute Inc., USA 2002). Data were found to be normally distributed and expressed as mean±SD. Comparisons between 2 groups were analyzed by Student t test. Spearman correlation was used to determine relations between parameters. A value of p<0.05 was considered statistically significant.
RESULTS
Matrix-embedding represses the induction of MHC class II expression by IFN-γ
Surface expression of MHC class II molecules on endothelial cells was determined via flow cytometry. Resting HAE did not express MHC class II, whereas treatment with IFN-γ induced its expression on endothelial cells (Fig. 1A). In matrix-embedded HAE, MHC class II induction by IFN-γ was significantly reduced by 45%. RT-PCR analysis revealed decreased MHC class II (HLA-DRα) mRNA transcript levels after IFN-γ stimulation of matrix-embedded HAE compared to HAE grown on tissue culture plates (0.11±0.08 vs. 0.29±0.11 DRα/GAPDH ratio, p<0.0005; Fig. 1B). HLA-DRα mRNA transcript levels significantly correlated with surface expression of MHC class II molecules on free and matrix-embedded HAE (r=0.57; p<0.05; Fig. 1C).
Figure 1. Matrix embedding attenuates IFN-γ induced endothelial MHC class II expression.
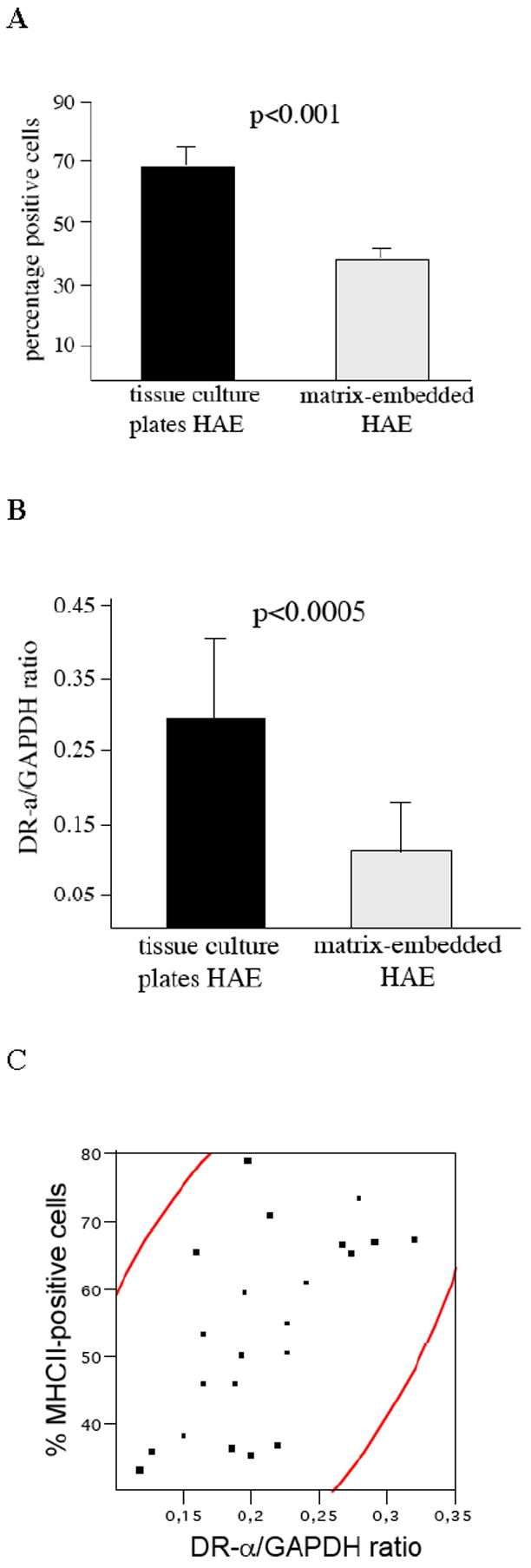
A 104 endothelial cells were analyzed for surface expression of MHC class II expression via flow cytometry.
B RT-PCR analysis revealed reduced mRNA transcript levels of HLA-DRα in matrix-embedded HAE.
C Spearman correlation of HLA-DRα mRNA transcript levels and MHC class II surface expression on HAE. Area of the density ellipse represents the 95% confidence interval.
Substrate-adherence does not influence IFN-γ surface receptor expression but intracellular signaling
No difference was detected in staining intensity of IFN-γ-receptor subunits I and II (Fig. 2A) in HAE for the two cell vehicles under confocal immunofluorescence microscopy. IFN-γ receptor subunits appeared more centralized in HAE substrate-adherent to a three-dimensional Gelfoam matrix. No significant differences in IFN-γR chain 1 (84.4±4.5 vs. 82.3±8.5% positive HAE; p=0.39) and chain 2 (80.9±9.1 vs. 79.1±4.5 positive HAE; p=0.69) surface expression were observed under flow cytometry between HAE grown on tissue culture polystyrene plates and embedded within three-dimensional Gelfoam scaffolds (Fig. 2B). Yet, Western blot analysis using an antibody against phospho-tyrosine-specific STAT-1 showed weaker and curtailed STAT-1-phosphorylation in matrix-embedded HAE when compared to HAE grown to confluence on tissue culture plates (Fig. 2C). There was no significant difference in concentration of unphosphorylated STAT-1 between the two groups of endothelial cells (data not shown). Furthermore, culture on collagen-coated tissue culture plates did not affect STAT-1 phosphorylation upon IFN-γ stimulation of HAE (data not shown).
Figure 2. Though expression of IFN-γ receptors was similar in HAE grown on TCPS or cultured within a three-dimensional matrix, matrix-embedding attenuates intracellular signaling pathways downstream of IFN-γ binding to its receptor.
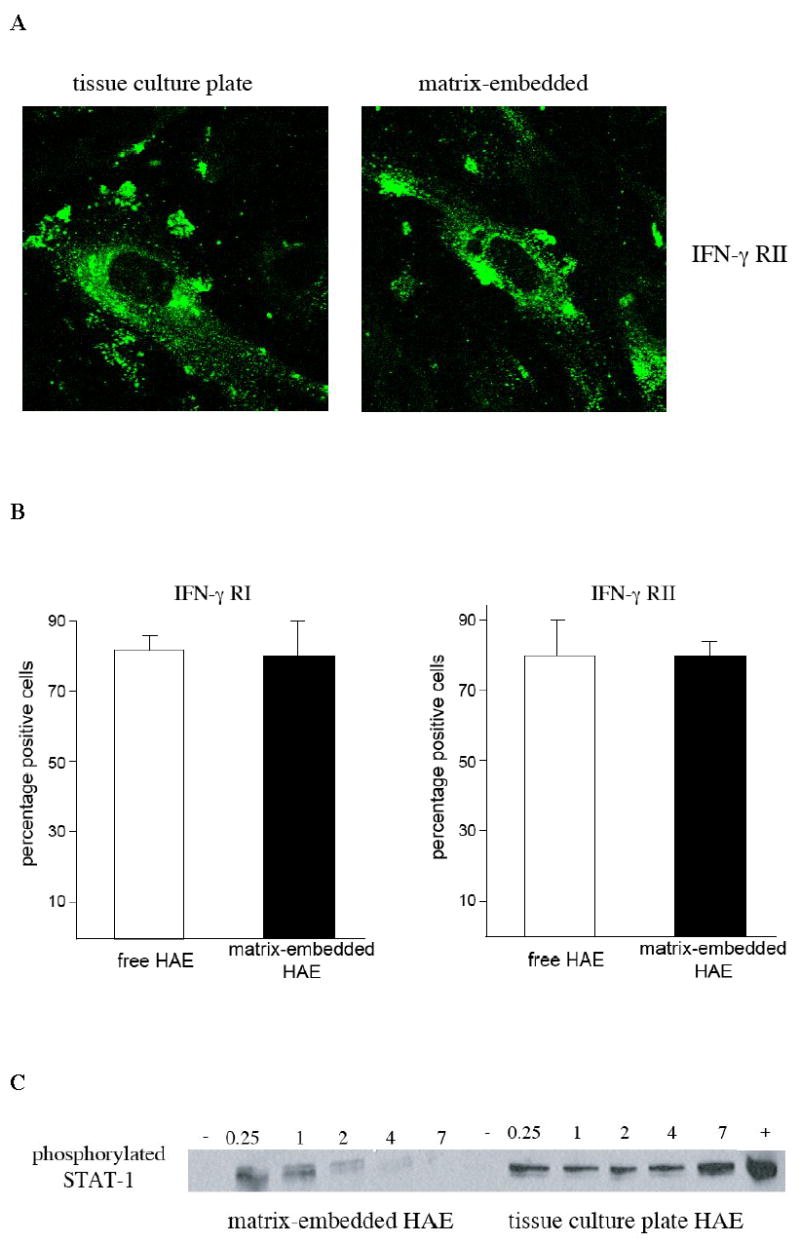
A IFN-γ receptor subunit II analyzed by immunofluorescence confocal microscopy (100X).
B Matrix-embedding has no influence on endothelial expression of IFN-γ as revealed via flow cytometry for IFN-γ receptor chain 1 and 2.
C Western blot demonstrated attenuated phosphorylation of STAT-1 after IFN-γ stimulation for indicated time periods in matrix-embedded HAE when compared to HAE grown to confluence on tissue culture polystyrene plates.
Matrix-embedded endothelial cells exhibit an altered integrin expression
Microarray analysis revealed significant differences in integrin expression between matrix-embedded endothelial cells and endothelial cells grown to confluence on tissue culture polystyrene plates (Fig. 3A). RT-PCR confirmed higher expression of integrins α1, α2b, αV, and β3 on endothelial cells grown on tissue culture plates (p<0.005) whereas matrix-embedded endothelial cells displayed higher expression of αX and β4 (p<0.02; Fig. 3B). Expression of αV (r=0.81, p<0.02) and β3 (r=0.63, p<0.05) significantly correlated with surface expression of MHC class II molecules on free and matrix-embedded HAE (data not shown). There was no significant difference between polystyrene tissue culture plates and collagen-coated tissue culture plates (Fig. 3B).
Figure 3. Matrix-embedded endothelial cells exhibit an altered integrin expression .
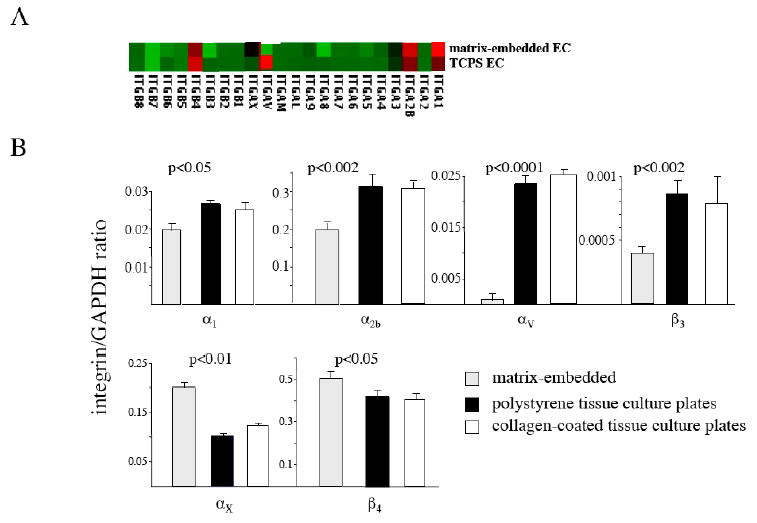
A Microarray analysis (A) and RT-PCR (B) revealed significant differences in integrin expression between matrix-embedded endothelial cells and endothelial cells grown to confluence on tissue culture polystyrene plates.
Splenocyte proliferation assay
The proliferative response of porcine splenocytes to untreated and IFN-γ treated (1000 U/ml, 48 hours) matrix-embedded, or tissue culture plate-cultured, HAE was assayed by thymidine incorporation. Non-stimulated HAE induced only a weak splenocyte proliferation. The strong splenocyte proliferation noted after exposure to HAE pretreated with IFN-γ was nearly eliminated when HAE were matrix-embedded (19619.5±327.5 vs. 4152.8±255.3 cpm, p<0.001; Fig. 4). The presence of MHC II antibody blocked lymphocyte proliferation in response to IFN-γ-treated HAE by 95% comparable to baseline levels.
Figure 4.
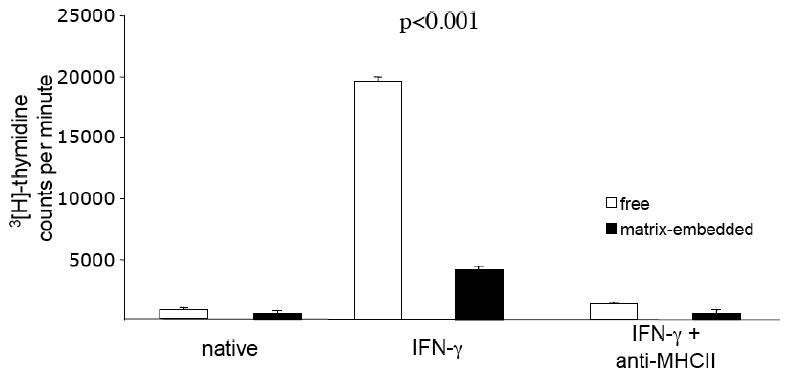
Unstimulated HAE did not evoke xenogeneic splenocyte proliferation. HAE-stimulation with IFN-γ resulted in a significant proliferative response of splenocytes, which was significantly greater against HAE grown to confluence on tissue culture plates compared to matrix embedded cells. The presence of MHC II antibody blocked splenocyte proliferation in response to IFN-γ treated HAE. Each value represents mean±SD.
DISCUSSION
Tissue engineering enables creation of an artificial endothelium that can be quantitatively characterized, maintained stably, and then transported and implanted where desired. In the perivascular vicinity of diseased vessels such constructs enhance healing without restoration of the natural endothelial monolayer that should reside at the luminal:mural interface and even with allo- and xenogeneic endothelial cells. We now extended previous investigations on alterations of endothelial immunogenicity with respect to the microenvironment.14 Endothelial cell-embedding within a three-dimensional environment influences intracellular signaling. Matrix-embedded HAE express significantly lower protein and mRNA MHC II levels upon stimulation with IFN-γ than HAE grown on tissue culture polystyrene plates. This effect correlated with muted proliferation of xenogeneic splenocytes to matrix-embedded HAE, whereas proliferation of splenocytes to HAE grown on tissue culture plates could be inhibited by addition of MHC II antibodies.
While we could not detect any differences in IFN-γ receptor surface expression, the effect of IFN-γ binding to its receptor on phosphorylation of the signaling factor STAT-1 was significantly weaker and of shorter duration in matrix-embedded HAE compared with HAE grown to confluence on tissue culture plates. Collagen coating of tissue culture plates was without effect on IFN-γ induced STAT-1 phosphorylation indicating a pivotal role of the microenvironment for endothelial immunogenicity. Inhibition of MHC II induction by IFN-γ via inhibition of STAT-1 phosphorylation occurs in tumor cells16 and an effect of matrix environment on MHC II expression in human fibroblasts has previously been published.17 Further analysis revealed differences in integrin expression patterns between endothelial cells grown within a three-dimensional matrix and on tissue culture plates. It is already established that integrin-binding to the endothelial basement membrane controls EC adhesion, spreading, migration, contractility, differentiation, proliferation, protein synthesis and secretion.18 As one consequence, deposition of transitional extracellular matrix proteins such as fibronectin and fibrinogen within the subendothelial matrix, as well as, detachment of EC from the basement membrane affects intra-EC signaling.19–21 Most strikingly endothelial cells grown to confluence on polystyrene tissue culture plates exhibited increased expression of integrins αV and β3 when compared with HAE embedded within Gelfoam matrices (Fig 3). Expression of integrin αV and β3 highly correlated with expression of MHC II on HAE. A pivotal role for these two integrins for cellular immune behavior22 may well explain the differences in proneness to cytokine-induced EC activation with respect to the matrix microenvironment. Further and more detailed investigation aims to identify the direct relationship between microenvironment, integrin expression and influences on intracellular signaling pathways.
Endothelial cell biosecretory function is density dependent. Our results now indicate the profound importance of matrix architecture and spatial environment for endothelial immunogenicity. These interactions are not only evidenced at the molecular and intracellular signaling levels, but also impact communication of EC with immune cells. By limiting splenocyte proliferation one can speculate that an effect on T cell memory function might even confer acceptance of secondary cell implants from the same donor (unpublished results). This is of special importance since the endothelial lining of grafts is the first line of contact with circulating host immune cells and might open new aspects for therapeutic alternatives with tissue engineered cellular constructs. Embedding endothelial cells within a three-dimensional matrix offers a tool not only to increase our understanding of the pathophysiology of diseased endothelium but also for the design of tissue engineered cellular constructs.
Footnotes
Financial support: Heiko Methe was supported by a grant from the Deutsche Herzstiftung, Frankfurt, Germany. Elazer Edelman was supported by a grant from the USA National Institutes of Health (HL 49309). Research described in this article was also supported by a grant Philip Morris USA Inc. and by Philip Morris International.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cortesini R. Progress in tissue engineering and organogenesis in transplantation medicine. Exp Clin Transplant. 2003;1:102. [PubMed] [Google Scholar]
- 2.Giannoudis PV, Pountos I. Tissue regeneration. The past, the present and the future. Injury. 2005;36:S2. doi: 10.1016/j.injury.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg LM, Eisenberg CA. Embryonic myocardium shows increased longevity as a functional tissue when cultured in the presence of a noncardiac tissue layer. Tissue Eng. 2006;12:835. doi: 10.1089/ten.2006.12.853. [DOI] [PubMed] [Google Scholar]
- 4.Korossis S, Bolland F, Ingham E, et al. Review: tissue engineering of the urinary bladder: considering structure-function relationships and the role of mechanotransduction. Tissue Eng. 2006;12:635. doi: 10.1089/ten.2006.12.635. [DOI] [PubMed] [Google Scholar]
- 5.Yates KE, Allemann F, Gowacki J. Phenotypic analysis of bovine chondrocytes cultures in 3D collagen sponges: effect of serum substitutes. Cell Tissue Bank. 2005;6:45. doi: 10.1007/s10561-005-5810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron DF, Hushen JJ, Colina L, et al. Formation and structure of transplantable tissue constructs generated in stimulated microgravity from Sertoli cells and neuron precursors. Cell Transplant. 2004;13:755. doi: 10.3727/000000004783983431. [DOI] [PubMed] [Google Scholar]
- 7.Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circ Res. 2003;92:1068. doi: 10.1161/01.RES.0000073844.41372.38. [DOI] [PubMed] [Google Scholar]
- 8.Nugent HM, Edelman ER. Endothelial implants provide long-term control of vascular repair in a porcine model of arterial injury. J Surg Res. 2001;99:228. doi: 10.1006/jsre.2001.6198. [DOI] [PubMed] [Google Scholar]
- 9.Nugent HM, Groothuis A, Seifert P, et al. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: a safety and efficacy study in the pig. J Vasc Res. 2002;39:524. doi: 10.1159/000067207. [DOI] [PubMed] [Google Scholar]
- 10.Nugent HM, Rogers C, Edelman ER. Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ Res. 1999;84:384. doi: 10.1161/01.res.84.4.384. [DOI] [PubMed] [Google Scholar]
- 11.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci U S A. 1995;92:8130. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 13.Dodge A, Lu X, D’Amore PA. Density-dependent endothelial cell production of an inhibitor of smooth muscle cell growth. J Cell Biochem. 1993;53:21. doi: 10.1002/jcb.240530104. [DOI] [PubMed] [Google Scholar]
- 14.Methe H, Nugent HM, Groothuis A, et al. Matrix embedding alters the immune response against endothelial cells in vitro and in vivo. Circulation. 2005;112:I89. doi: 10.1161/01.CIRCULATIONAHA.105.524991. [DOI] [PubMed] [Google Scholar]
- 15.Miller DM, Rahill BM, Boss JM, et al. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferry B, Halttunen J, Leszcynski D, et al. Impact of class II major histocompatibility complex antigen expression on the immunogenic potential of isolated rat vascular endothelial cells. Transplantation. 1989;44:499. doi: 10.1097/00007890-198710000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kern A, Liu K, Mansbridge J. Modification of fibroblast gamma-interferon responses by extracellular matrix. J Invest Dermatol. 2001;117:112. doi: 10.1046/j.0022-202x.2001.01386.x. [DOI] [PubMed] [Google Scholar]
- 18.Berman AE, Kozlova NI, Morozevich GE. Integrins: structure and signaling. Biochemistry (Mosc) 2003;68:1284. doi: 10.1023/b:biry.0000011649.03634.74. [DOI] [PubMed] [Google Scholar]
- 19.Orr AW, Sanders JM, Bevard M, et al. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 21.Sechler JL, Corbett SA, Wenk MB, et al. Modulation of cell-extracellular matrix interactions. Ann N Y Acad Sci. 1998;857:143. doi: 10.1111/j.1749-6632.1998.tb10114.x. [DOI] [PubMed] [Google Scholar]
- 22.Sahni A, Sahni KS, Francis CW. Endothelial cell activation by Il-1ß in the presence of fibrinogen requires αvβ3. Arterioscler Thromb Vasc Biol. 2005;25:2222. doi: 10.1161/01.ATV.0000183605.27125.6f. [DOI] [PubMed] [Google Scholar]


