Summary
Long (LMS) versus brief (BMS) daily separations of rat pups from their mothers have contrasting effects on their adult stress responses and maternal behavior by respectively decreasing and increasing licking received from their mothers. We hypothesized that LMS decreases pup licking in mothers by inducing learned helplessness, creating a depression-like state. We subjected postpartum rats to LMS (3 h), BMS (15 min) or no separation (NMS) on postpartum days 2–14. After weaning, mothers were given a forced swim test (FST). LMS mothers exhibited more immobility and fewer escape attempts than BMS or NMS mothers. These results suggest that LMS induces a depression-like state, which may account for the reductions in maternal behavior seen in LMS mothers. Immobility in the FST is recognized as an animal model of depression. Therefore, LMS may be a model of maternal depression.
Keywords: Maternal depression, learned helplessness, handling effects, maternal separation
Introduction
Syndromal depression is by far the most common serious medical complication of pregnancy and the postpartum period. A recent comprehensive review of the highest quality studies published over the past several decades funded by the U.S Department of Health and Human Services' Agency for Healthcare Research and Quality (Gaynes et al, 2005) concludes that the incidence of new depressive episodes meeting DSM-IV major or RDC minor criteria within the first 3 months postpartum may be as high as 14.5%. This is similar to the conclusion of an earlier meta-analysis of the literature that syndromal depression occurs in approximately 13% of women during the first several months postpartum (O'Hara and Swain, 1996). O'Hara et al. (1990) found that rates of major and minor depression were higher in parturient than nonparturient women, although not significantly. However, a significantly higher percentage of postpartum women showed elevated depression scores and poor social adjustment compared to nonparturient women.
Maternal depression affects children in multiple domains, including emotional, cognitive and physiological. Among effects on socioemotional development are behavior problems, reduced positive affect expression, difficulty with emotion regulation, and social impairments. Emotional effects include increased negativity and fearfulness (Pauli-Pott et al., 2004) and behavior problems (Beck, 1999) as well as more insecure attachments (Cicchetti et al., 1998). Cognitive effects on children experiencing maternal depression have been documented across childhood (Brennan et al. 2000; Cogill et al., 1986; NICHD Early Child Care Research Network 1999). Physiological consequences for children of depressed mothers include less weight gain in the first 2 yr of life (Hendrick 2003; O'Brien et al., 2004), elevated cortisol and norepinephrine, and lower dopamine levels (Diego et al 2004; Halligan et al., 2004). Unfortunately, there are at present no animal models of maternal depression.
Animal models of depression have taken advantage of the similarities between the behavioral consequences of chronic, inescapable and/or unpredictable stressors and depression. Stressors have included primarily physical stressors such as restraint, foot shock, active or passive avoidance tasks, water restraint, thermal extremes, pharmacological agents and surgical procedures, but also social stressors such as social defeat (Newport et al 2002; Benmansour et al 1999; Lucki et al., 2001; Malatynska et al 2002; Cryan et al., 2002; Ripoll et al., 2003; Lucassen et al., 2004). The Forced Swim Test (FST) is one model of inescapable stress and depression-like behavior (Porsolt et al., 1977; Yadid et al., 2001). Validation of this test includes successful treatment with antidepressants (Benmansour et al 1999; Lucki et al., 2001; Malatynska et al 2002; Cryan et al., 2002; Ripoll et al., 2003; Lucassen et al., 2004). Immobility, the behavioral marker of depression-like state in this model, decreases following treatment with antidepressants.
Unfortunately, non-primate models of depression involve primarily physical stressors or social stressors involving aggression, rather than disruption of social relationships. Human depression more often entails loss of, disruption of, or change in significant social relationships. Thus, an homologous animal model would be one based on depression-like responses to social relationship disruptions. The effects of maternal separation on infants and their later development has been used extensively in primates as a paradigm for studying bereavement, loss and depression (Seay et al., 1962; Kaufman & Rosenblum 1969; Suomi et al., 1976; Hinde et al., 1978; Laudenslager et al., 1982; Capitanio et al., 1986; Kalin & Takahashi 1988; Coe et al., 1989; Reite et al., 1989; Laudenslager et al 1990; Weiner et al., 1990; Boccia et al., 1991a; Boccia et al., 1991b; Reite et al., 1991). This has been a valuable animal model that has provided important information on separation-induced depression effects on the course of behavior responses, adult sociality, short- and long-term changes in immune measures, sleep disturbances, hormone and neurotransmitter disturbances and effects of antidepressants. Unlike human mothers, however, non-human primate mothers do not typically evidence depressive responses after separation from their infants. Although occasionally such a response is seen (e.g., Rasmussen & Reite 1982), inability to reliably induce depression in monkey mothers eliminates separation from infants as a useful model of postpartum depression and maternal depression in general.
Repeated maternal separations during the postpartum period have profound effects on rats when they reach adulthood (Levine 1957; 1962; Denenberg 1964; Levine et al 1967; Ader & Grota, 1969; Plotsky & Meaney, 1993; Ogawa et al 1994; Ladd et al 1996; Lui et al 1997; Caldji et al 1998; Boccia & Pedersen 2001; Gonzalez et al 2001; Ogawa et al 1994). Brief (approximately 15 min) maternal separations (BMS) have contrasting effects from long 3 to 6h separations (LMS). These effects have been found on hormonal and behavioral responses to stressors, maternal behavior and aggression, and neurochemical systems regulating these areas. Mothers who are separated from their pups for brief daily periods have been shown to increase their maternal behavior, especially pup-licking, while mothers separated for long periods have been shown to decrease their maternal behavior (Boccia & Pedersen, 2001; Lovic et al 2001). Remarkably, the impact of repeated separations on rat mothers' emotionality has not been studied at all.
We hypothesized that repeated long separations from pups, may produce a depression-like state of learned helplessness in rat mothers. With repeated separations, the rat mother may learn that it cannot control access to its pups, therefore efforts to do so and to exhibit active maternal behaviors such as pup-licking decline. Induction of learned helplessness in this way may induce a depression-like state in the rat mother. We tested our hypothesis by measuring FST performance shortly after weaning in rat mothers that had been subjected to brief, long or no separation from pups on postpartum days 2–14.
Methods
Subjects
Three shipments of 20 pregnant Long Evans rats were obtained from Charles River (Raleigh NC) and arrived in the lab on pregnancy day 15. Each cohort of females was impregnated on the same day and arrived in our laboratory on gestation day 15. Females in each cohort that gave birth on the same day were used in this study.
Procedures
At 1000h on the day after each wave of mothers gave birth (postpartum day 2), birth litters were removed from their mothers' cages and male and female pups in each litter were separated. Experimental litters composed of 5 male and 5 female pups were then formed by taking each pup from a different birth litter. Fifteen mothers (5 per cohort) were randomly assigned to each of the 3 maternal separation groups (see description below). In each cohort, females in excess of the 15 experimental females ensured adequate numbers of synchronous births, and provided pups to permit composition of litters of equal numbers, balanced sex distribution, and unrelated pups to each mother. Each experimental mother was given an experimental litter that contained none of her own birth pups and no pups related to each other.
Mothers assigned to the no maternal separation (NMS) or brief maternal separation (BMS) groups were given experimental litters as soon as they were formed which was approximately 45–60 min after removal of their birth litters. Mothers assigned to the long maternal separation (LMS) group were given their experimental litters at 1300h. On subsequent postpartum days (3–14), mothers assigned to the BMS group were separated from their litters for 15 min beginning at 1245h, mothers assigned to the LMS group were separated from their litters for 3 h beginning at 1000h, and mothers assigned to the NMS group were not separated from their pups or disturbed at all. Separations involved first removing each mother from her home cage to her own holding cage where she remained for the entire duration of that day's separation. Each litter was then removed from its maternal cage and placed as a group in its own small holding cage in an Ohmeda incubator set at 32°C. Holding cages for mothers and litters and the bedding in those cages were not changed during the repeated separation period. At the end of the separation period, the litter was returned to the home cage and pups were distributed evenly over the floor of the cage. Mothers were then returned to the center of their home cages. Routine cage maintenance was suspended from 2 days prior to parturition until postpartum day 15 when twice weekly cage and bedding changes resumed.
On postpartum days 2 through 6, mother-pup interactions were videotaped for 4h during the light phase beginning at 1430h (0700–1900h lights on) and 4h during the dark phase from 2230 to 0230. A high resolution, low light Panasonic camera was focused on each maternal cage, so that the image of the cage filled the entire field of view. A mirror the same length as the cage was placed behind the cage at an angle so that the behavior of the mothers could be observed even when they were facing away from the camera.
Pups were permanently removed from dams' cages the morning of postpartum day 21. The pups entered other experimental protocols at this time. Mothers were subjected to the FST that afternoon and the following day. Because the pups were targeted to enter other experimental protocols, the FST was scheduled for the day after weaning, so as to ensure no impact on the pups. The FST was conducted in a cylindrical tank (40 cm high and 18 cm in diameter) constructed of clear Plexiglas (Overstreet, 2002). The tank was filled with water (25°C) to a depth that rats could not touch the bottom with their hind paws. On day 1, mothers were tested in the swim tank for 15 min. Twenty-four h later, they were tested for 5 min. Behavior in the swim tanks was videotaped on both days with Panasonic VHS camcorders placed approximately 1.5 m from the tanks.
Both days' FST tapes were subsequently scored using The Observer (Noldus Information Technology, 2002) to score real-time duration and frequency of behavior. Behaviors observed included immobility (cessation of swimming except for small movements required to stay afloat), swimming (any greater movement) and escape attempts (swimming to the bottom of the tank and pushing off). Mean percentages of time spent immobile and numbers of escape attempts were used in subsequent analysis.
Mothers' frequency of pup-licking was scored during 10s intervals every 4min from the 4h videotapes recorded on postpartum days 2–6. Videotapes were reviewed on a large 24" Panasonic monitor that allowed larger-than-life-size viewing of the behavior. Observer reliability was established at 90% or greater before scoring tapes for analysis.
Mothers that did not deliver on the targeted day, killed pups or lost 3 or more pups for other reasons before weaning were not included in this study. Because of this attrition and some technical errors, the final sample included 35 mothers, 12 BMS, 13 LMS and 10 NMS.
Data Analysis
Behaviors during the FST and pup-licking during postpartum day 2–6 were analyzed using Analyses of Variance (ANOVAs) and CoVariance (ANCOVA) with separation condition (NMS, BMS, LMS) as a between subjects variable and test day as a within subjects variable. Pup licking was examined as an independent behavior. Significant effects were further investigated with Bonferroni post-hoc tests. Relationships between FST data and pup-licking behavior were analyzed using Pearson Product Moment correlations.
Results
Immobility in the Forced Swim Test
Immobility in the FST was significantly affected by separation experience (Main Effect of separation experience: F(2/35)= 7.629, P=.002, see Figure 1). Mothers that had been repeatedly separated from their pups for 3h per day (LMS) showed significantly higher percentage of immobility in the FST than mothers subjected to NMS or BMS, indicating greater depression-like response. This significant difference was seen on both the first and second day of the FST (separation condition X day of test interaction, ns). This suggests that the experience of repeated long separations from pups may have induced a depression-like learned helplessness state rather than rendering mothers more vulnerable to induction of learned helplessness by the first day's inescapable swim stress experience.
Figure 1.
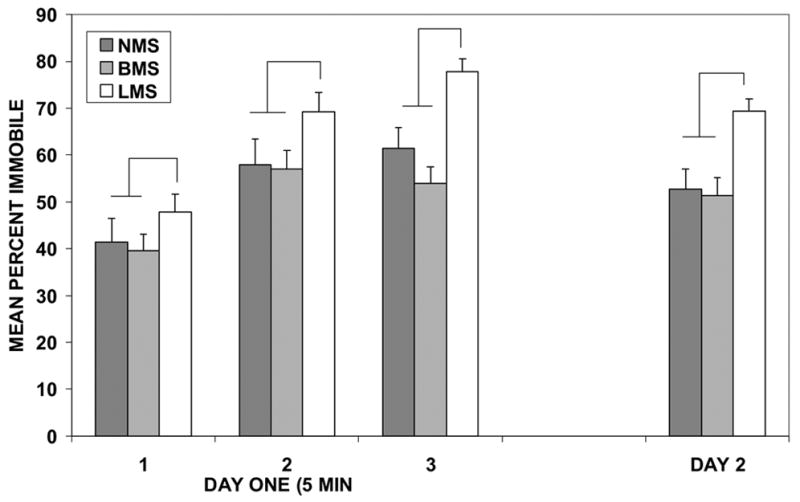
Mothers' immobility in the FST. Day 1 results are divided into three 5-min blocks. Mothers who experienced long separations from their pups (LMS) exhibited greater immobility in the FST at weaning. Lines indicate significant main effect that percent time immobile was greater in LMS than the other two groups on both the first and second days of testing.
Escape attempts by the rats were significantly higher on the first day of testing than the second (F(1/35)=4.349, p=.044, figure 2). On the first day of testing, the groups did not differ in escape attempts (F(2/35)=0.818 ns). However, on the second day of testing, escape attempts were significantly affected by separation experience (F(2/37)=3.693, p=.035). Mothers that had been subjected to LMS made significantly fewer escape attempts on the second day of the FST than mothers subjected to NMS or BMS (see figure 2).
Figure 2.
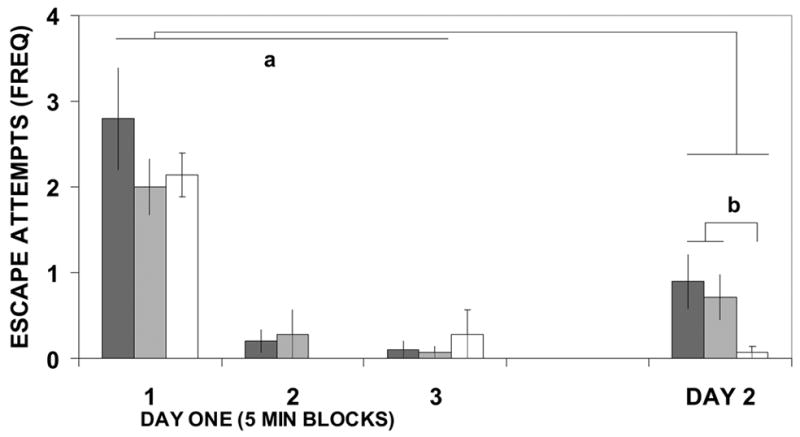
Mothers' escape attempts in the FST. Day 1 divided into 3 5-min blocks. Legend as in figure 1. Lines indicated by "a" highlight the main effect that all animals exhibited more escape attempts on Day 1 than Day 2. Lines indicated by "b" highlight that mothers who experienced long separations from their pups exhibited fewer escape attempts during day 2 of the FST.
Relationship between immobility and maternal behavior
There was a trend for mean pup-licking on post-partum days 2–6 to be statistically different between separation groups (F(2/35) = 2.739, p=.07). Mothers in the LMS group (M = 6.72 ± .83 SEM) tended to lick their pups less than mothers in the NMS or BMS separation conditions (M = 8.66 ± .53 and M = 8.34 ± .28, respectively).
A negative relationship was found between maternal immobility on day 2 of the FST and maternal pup-licking behavior on postpartum days 2–6. Immobility in the FST correlated significantly with pup-licking frequencies (r= −.41, p=.01, 33 df, see figure 3) such that higher rates of pup-licking were associated with less immobility on day 2 of the FST. Correlations with immobility during the 5 min blocks of day 1 of the FST were also negatively correlated with pup-licking on postpartum days 2–6, although not significantly so (first block r= −.310 p=.06; second block r=−.153, ns; third block r=−.270, p=.11). When the groups were examined separately, these correlations remained negative, although not significantly, for NMS (r= −.376) and LMS (r=−.419), probably due to reduced power. Correlations between immobility on day 2 and pup-lick for BMS mothers was near zero. Similar patterns of correlation for immobility during the 5 min blocks on day 1 and pup-lick were found for the separate groups.
Figure 3.
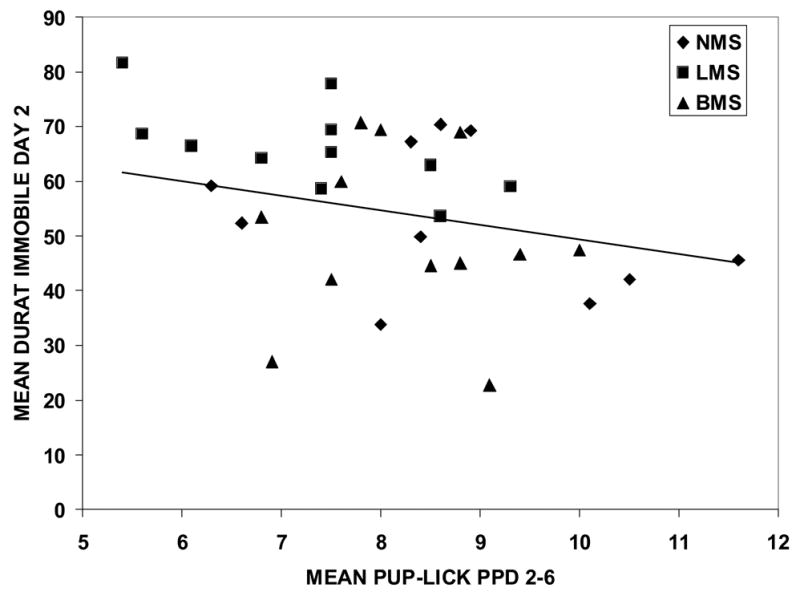
Relationship between mothers' pup licking and immobility on the second day of the FST. Mothers' pup-licking on postpartum days 2–6 was negatively correlated with immobility in the FST (r=−.41, p=.01). Separation experience of each individual is distinguished by symbols, as indicated in the graph.
Discussion
Repeated, long separations of rat mothers from their pups increased immobility and decreased escape attempts during the FST performed at weaning, 1 wk after the last separation from their pups. These effects were evident on the first day of the test, which suggests that LMS experience actually induced depressive responses (rather than increased vulnerability to induction of depressive response to the inescapable test), which was then exhibited in the FST. Others have found FST differences on the first day of testing when comparing selected rat strains or in animals with genetic alterations or neonatal manipulations (Keck et al 2001; Bulduk & Canbeyli 2004). Other manipulations, such as inescapable shock (Yilmaz et al 2002) or lesion studies (Tataroglu et al 2004), produce differences on day 2 but not day 1 of the FST. This suggests there may be two, separable forms of depression, one related to genetic or early experience-induced vulnerability that results in rapid onset "hopelessness" when initially exposed to stress and another produced by chronic, inescapable and/or unpredictable stressors experienced in adulthood that results in a vulnerability to emergence of hopelessness when again subjected to subsequent repeated inescapable stressors. The results reported here represent a unique outcome in which the first form of depression, rapid onset, is induced by disruptions of attachment relationships in an adult (mothers to pups). These animal models may provide important information about the similarities and differences in the underlying pathology of these two forms of depression.
The effects of LMS experience on rat mothers' FST performance may be the first rodent model of depression that is induced solely by disruption of a social relationship. Other rodent models of learned helplessness/depression involve exposure to chronic, inescapable or unpredictable physical stress. Social stressor models have focused on aggression and social defeat. The effects of LMS described here appear to be a closer approximation than other rodent models to human and nonhuman primate depressive states produced by repeated or permanent separations of closely attached individuals. In particular, human maternal depression has been associated with, among other things, a loss of control (Beck 2002), which may therefore make a learned helplessness model of maternal depression particularly useful. This model, therefore, needs further characterization and validation, in terms of other components of human depression, including anhedonia, HPA hyperactivity and response to antidepressant treatment. Validation would warrant detailed investigation of the neurobiology of LMS induced maternal depression-like behavior.
It is also of great interest that FST performance was related to maternal pup-licking, which has profound effects on the development of stress responses in offspring (Caldji et al 1998; Liu et al 1997; see also Pedersen & Boccia 2002). FST immobility correlated negatively with pup-licking across all mothers, regardless of separation history, and for both days of the FST. This suggests that depression-like states may contribute to the level of expression of maternal behavior.
There are some controversies in the interpretation of both the FST and the maternal separation/pup-licking in the literature that suggest caution in the interpretation of these results. There has been some discussion in the literature regarding whether immobility in the FST is an indicator of despair or learned helplessness or whether it is an indicator of passive, in contrast to active, coping (Cryan et al 2002, 2005). This distinction, however, may also be applied to human depression, particularly anaclitic depression, where reduced activity has be interpreted as an energy conserving coping response (e.g., Kaufman & Rosenblum, 1969). With regard to maternal separation, there has been discussion about the suspension or continuation of normal husbandry during the separation period, particularly with regard to the meaning of the unseparated condition as a control group (e.g., Pryce & Feldon, 2003). While the impact of separation on pup's later stress responses in adulthood appear to be complexly related to maternal behavior, and husbandry practices, the focus of this study was on the impact of maternal separation on the mothers. The notion of control group is a significant one, and we chose to focus on separation per se, and included a no-separation group which we referred to as NMS. Whether the presence or absence of husbandry impacts the mother's responses to separation during the first two weeks of their pups' lives is unknown at the present time.
Syndromal (major or minor) depression appears to be at least as prevalent during the postpartum period as syndromal depression in the general population of women. Debilitating subsyndromal depressive symptoms, however, are more common in new mothers (O'Hara et al., 1990). While depression has profound consequences for the women experiencing it, maternal depression has the added complication of negative outcomes for children of these women (Beck, 1999; Cicchetti et al., 1998; Pauli-Pott et al., 2004). The effects of LMS on FST performance presented here may be of great utility in providing a model system in which to study both the immediate and long-term consequences of postpartum depression on both mothers and offspring.
Acknowledgments
This research was supported by PHS Grants MH066217 to MLB and MH61995 to CAP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, Grota LJ. Effects of early experience on adrenocortical reactivity. Physiology & Behavior. 1969;4:303–305. [Google Scholar]
- Beck CT. Maternal depression and child behaviour problems: A meta-analysis. Journal of Advanced Nursing. 1999;29:623–629. doi: 10.1046/j.1365-2648.1999.00943.x. [DOI] [PubMed] [Google Scholar]
- Beck CT. Postpartum depression: A metasynthesis. Qualitative Health Research. 2002;12:453–472. doi: 10.1177/104973202129120016. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. Journal of Neuroscience. 1999;19(23):10494–501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separation in infancy: Contrasting relationships with adult maternal behavior, lactational levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Reite ML, Laudenslager ML. Early social environment may alter the development of attachment and social support: Two case reports. Infant Behavior and Development. 1991a;14:253–260. [Google Scholar]
- Boccia ML, Laudenslager ML, Reite ML. Heart rate level predicts biobehavioral response to separation in pigtail monkey infants (Macaca nemestrina) American Journal of Primatology. 1991b;24:90–91. [Google Scholar]
- Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM. Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Developmental Psychology. 2000;36(6):759–766. doi: 10.1037//0012-1649.36.6.759. [DOI] [PubMed] [Google Scholar]
- Bulduk S, Canbeyli R. Effect of inescapable tones on behavioral despair in Wistar rats. Progress in Neuropsychopharmacology & Biological Psychiatry. 2004;28:471–5. doi: 10.1016/j.pnpbp.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Science USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Rasmussen KLR, Snyder DS, Laudenslager M, Reite M. Long-term follow-up of previously separated pigtail macaques: Group and individual differences in response to novel situations. Journal of Child Psychology and Psychiatry. 1986;27:531–538. doi: 10.1111/j.1469-7610.1986.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: Contributions to the development of attachment insecurity and behavior problems in toddlerhood. Developmental Psychopathology. 1998;10(2):283–300. doi: 10.1017/s0954579498001618. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach G, Ershler WB. Immunological consequences of maternal separation in infant primates. New Directions for Child Development. 1989;45:65–91. doi: 10.1002/cd.23219894507. [DOI] [PubMed] [Google Scholar]
- Cogill SR, Caplan HL, Alexandra H, Robson KM, Kumar R. Impact of maternal postnatal depression on cognitive development of young children. British Medical Journal. 1986;292(6529):1165–1167. doi: 10.1136/bmj.292.6529.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Science. 2002;23(5):238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Critical periods, stimulus input, and emotional reactivity: A theory of infantile stimulation. Psychological Review. 1964;66:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67(1):63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. AHRQ Publication No. 05-E006-2. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence Report/Technology Assessment No. 119. (Prepared by the TRI-University of North Carolina Evidence-based Practice Center, under Contract No. 290-02-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Developmental Psychobiology. 2001;38(1):11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55(4):376–81. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hendrick V. Treatment of postnatal depression. BMJ. 2003;327(7422):1003–4. doi: 10.1136/bmj.327.7422.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde RA, Leighton ME, McGinnis I. Effects of various types of separation experience on rhesus monkeys five months later. Journal of Child Psychology and Psychiatry. 1978;19:199–211. doi: 10.1111/j.1469-7610.1978.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK. Altered hypothalamic-pituitary-adrenal regulation in animal models of depression. In: Schatzberg AF, Nemeroff CB, editors. The Hypothalamic-Pituitary-Adrenal Axis: Physiology, Pathophysiology, and Psychiatric Implications. New York: Raven Press, Ltd; 1988. pp. 67–78. [Google Scholar]
- Kaufman C, Rosenblum LA. Effects of separation from mother on the emotional behavior of infant monkeys. In: Tobach E, editor. Experimental Approaches to the Study of Behavior. New York: Annals of the New York Academy of Science; 1969. pp. 681–695. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Post A, Muller MB, Toschi N, Wigger A, Landgraf R, Holsboer F, Engelmann M. Neuroendocrine and behavioral effects of repetitive transcranial magnetic stimulation in a psychopathological animal model are suggestive of antidepressant-like effects. Neuropsychopharmacology. 2001;24:337–49. doi: 10.1016/S0893-133X(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Held PE, Boccia ML, Reite ML, et al. Behavioral and immunological consequences of brief mother-infant separation: A species comparison. Developmental Psychobiology. 1990;23(3):247–264. doi: 10.1002/dev.420230305. [DOI] [PubMed] [Google Scholar]
- Laudenslager M, Reite M, Harbeck R. Suppressed immune response in infant monkeys associated with maternal separation. Behavioral and Neurological Biology. 1982;36:40–48. doi: 10.1016/s0163-1047(82)90223-0. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;136:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiology & Behavior. 1967;2:55–59. [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Cladji C, Francis D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovic V, Gonzalez A, Fleming AS. Maternally separated rats show deficits in maternal care in adulthood. Developmental Psychobiology. 2001;39(1):19–33. doi: 10.1002/dev.1024. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155(3):315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Fuchs E, Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biological Psychiatry. 2004;55(8):789–96. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Malatynska E, Goldenberg R, Shuck L, Haque A, Zamecki P, et al. Reduction of submissive behavior in rats: a test for antidepressant drug activity. Pharmacology. 2002;64(1):8–17. doi: 10.1159/000056145. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: Animal models of an adverse life event. American Journal of Psychiatry. 2002;159:1265–1283. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Child Development. 1999;35(5):129–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- Noldus Information Technology. The Observer: Professional System for Collection, Analysis, Presentation and Management of Observational Data. Wageningen, The Netherlands: Noldus Information Technology, bv; 2002. [Google Scholar]
- O'Brien LM, Heycock EG, Hanna M, Jones PW, Cox JL. Postnatal depression and faltering growth: a community study. Pediatrics. 2004;113(5):1242–7. doi: 10.1542/peds.113.5.1242. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Swain AM. Rates and risk of postpartum depression-a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- O'Hara MW, Zekoski EM, Philipps LH, Wright EJ. Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. Journal of Abnorrnal Psychology. 1990;99:3–15. doi: 10.1037//0021-843x.99.1.3. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, et al. Periodic maternal deprivation alters stress response in adult offspring: Potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacology Biochemistry & Behavior. 1994;49:961–967. doi: 10.1016/0091-3057(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Behavioral characteristics of rat lines selected for differential hypothermic responses to cholinergic or serotonergic agonists. Behavior Genetics. 2002;32:335–348. doi: 10.1023/a:1020262205227. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, Mertesacker B, Beckmann D. Predicting the development of infant emotionality from maternal characteristics. Developmental Psychopathology. 2004;16(1):19–42. [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5(4):259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. 2003. [DOI] [PubMed] [Google Scholar]
- Rasmussen KLR, Reite M. Loss-induced depression in an adult macaque monkey. American Journal of Psychiatry. 1982;139:679–681. doi: 10.1176/ajp.139.5.679. [DOI] [PubMed] [Google Scholar]
- Reite M, Kaemingk K, Boccia ML. Maternal separation in bonnet monkey infants: Altered attachment and social support. Child Development. 1989;60(2):473–480. [PubMed] [Google Scholar]
- Reite ML, Cox M, Laudenslager ML, Garrick N, Boccia ML. Effects of monoamine oxidase-A inhibitor on maternal separation in pigtail monkey infants. American Journal of Primatology. 1991;24:120. [Google Scholar]
- Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behavior & Brain Research. 2003;143(2):193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Seay B, Hansen E, Harlow HF. Mother-infant separation in monkeys. Journal of Child Psychology and Psychiatry. 1962;3:123–132. doi: 10.1111/j.1469-7610.1962.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Collins ML, Harlow HF, Ruppenthal GC. Effects of maternal and peer separation on young monkeys. Journal of Child Psychology and Psychiatry. 1976;17:101–112. doi: 10.1111/j.1469-7610.1976.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Research. 2004;1001:118–24. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Weiner SG, Bayart F, Faull KF, Levine S. Behavioral and physiological responses to maternal separation in squirrel monkeys. Behavioral Neuroscience. 1990;104:108–115. doi: 10.1037//0735-7044.104.1.108. [DOI] [PubMed] [Google Scholar]
- Yadid G, Overstreet DH, Zangen A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Research. 2001;896:43–47. doi: 10.1016/s0006-8993(00)03248-0. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacology, Biochemistry & Behavior. 2002;71:341–4. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]


