Abstract
An adenoviral vector containing the muIFN-γ transgene (Ad:IFN-γ) was evaluated for its capacity to inhibit HSV-1. To measure effectiveness, viral titers were analyzed in cornea and trigeminal ganglia (TG) during acute ocular HSV-1 infection. Ad: IFN-γ potently suppressed HSV-1 replication in a dose-dependent fashion, requiring IFN-γ R. Moreover, Ad:IFN-γ was effective when delivered -72 and -24 h prior to infection as well as 24 h post infection. Associated with anti-viral opposition, TG from Ad: IFN-γ transduced mice harbored fewer T cells. Also related to T cell involvement, Ad:IFN-γ was effective but attenuated in TG from α/β TCR deficient mice. In corneas, α/β TCR+ T cells were obligatory for protection against viral multiplication. Type I IFN involvement amid anti-viral efficacy of Ad: IFN-γ was further investigated because type I and II IFN pathways have synergistic anti-HSV-1 activity. Ad:IFN-γ inhibited viral reproduction in corneas and TG from IFN-α/β R deficient (CD118 −/−) mice, although viral titers were 2–3 fold higher in cornea and TG, compared to wild type. The absence of IFN-stimulated anti-viral proteins, 2’-5’ oligoadenylate synthetase/RNase L and ds RNA dependent protein kinase R, completely eliminated the anti-viral effectiveness of Ad:IFN-γ. Collectively, the results demonstrate: (1) nonexistence of type I IFN R does not abolish defense of Ad:IFN-γ against HSV-1; (2) anti-viral pathways, OAS/RNase L and PKR are mandatory; and (3) α/β TCR+ T cells are compulsory for Ad: IFN-γ effectiveness against HSV-1 in cornea but not in TG.
Keywords: rodent, T cells, cytokine, knockout mice, viral
Introduction
IFN-γ is a potent endogenous anti-viral cytokine, made by leukocytes including T, NK, and NKT cells that operates through a single IFN-γ R (1, 2). Inhibition of neurotropic viruses by IFN-γ involves activation of double stranded RNA-dependent protein kinase (PKR)4 (3–5), oligoadenylate synthetases (OAS) (6, 7), Mx (8–10), and nitric oxide synthetase (11–13) anti-viral pathways (14). In contrast to these findings, adenoviral vector delivery of the IFN-γ transgene to explant trigeminal ganglion (TG) cultures inhibits acute HSV-1 replication independently of recognized strategies for other neurotropic viruses (15).
As a follow-up to prior in vitro studies, an objective of the current report was to examine how IFN-γ, delivered by an adenoviral vector (Ad:IFN-γ), inhibits acute ocular HSV-1 infection in vivo. Adenovirus is a useful vector for delivering IFN transgenes to the cornea and TG (16–18). To examine the mechanism(s) used by IFN-γ to inhibit HSV-1, we studied parameters including participation of type I IFN inducible anti-viral pathways, OAS and a downstream effector - RNase (OAS/RL), PKR, the need for type I and II IFN R expression, and the requirement for α/β TCR+ T cells.
A prediction for type I IFN involvement follows: in mice lacking type I IFN antiviral pathways, infectious HSV-1 will reach higher levels in cornea and TG, compared with wild type (WT) mice. We reasoned that since the IFN α/β system alone controls herpes infection for the first several days of infection without T, NK, or B cells(19), then viral burder will likely be greater in the absence of this protective mechanism.
Type I and II IFN anti-HSV activities have been linked. Specifically, type I and type II IFNs have synergistic anti-HSV-1 activity (20–22). In response to some viral infections, both IFN pathways are required and considered non-redundant (23). To examine the relationship of type I and II IFN anti-viral pathways during HSV-1 infection, the following hypothesis was tested: if type I IFN pathways are not required for Ad: IFN-γ effectiveness against acute HSV-1 infection, then transduction of IFN α/β R deficient (CD 118 −/−) mouse corneas with Ad:IFN-γ will provide protection against HSV-1 replication..
Downstream effector molecules of IFN inducible anti-viral pathways, OAS and PKR, have proven anti-viral activity. Yet, cells lacking OAS and PKR can still mount an anti-viral response (24), and effectiveness of Ad: IFN-γ in dissociated TG cultures is not lost in the absence of OAS/PKR (15). To resolve the necessity for OAS/PKR in vivo, we tested the hypothesis: if effectiveness of Ad:IFN-γ is independent of OAS/PKR, then the ability of Ad:IFN-γ transduction to inhibit viral replication will be lost in RL/PKR −/−mice.
In addition to effects on IFN-stimulated gene expression, the anti-viral outcome of Ad:IFN-γ transduction is anticipated to involve T cells. Specifically, T cells are found in TG during acute HSV-1 infection (25), and T cell deficient mice are highly susceptible to HSV-1 (26). T cells accumulate around neurons that are infected with HSV-1, and IFN-γ is present in the vicinity amid T cell infiltrates (27, 28). Therefore, the question arises: will protective anti-viral activity of Ad:IFN-γ be lost in the absence of α/β TCR+ T cells? We predict the dearth of α/β TCR+ T cells to impact anti-viral activity of Ad:IFN-γ for the following reasons: T cells are a source of anti-viral cytokines including TNF-α, which has supplementary anti-HSV-1 properties (29); and virus surveillance by T cells involves direct cytolytic action by effector T cells. Therefore, the following hypothesis was tested: if T cells are required for anti-viral effectiveness of Ad:IFN-γ, then anti-viral activity following Ad:IFN-γ transduction will be attenuated in mice lacking α/β TCR+ T cells, compared to WT mice.
Materials and Methods
Mice
Animal treatment was consistent with the National Institute of Health Guidelines on the Care and Use of Laboratory Animals. All procedures were approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute institutional animal care and use committees. WT (C57BL/6), CD119/IFN-γ R deficient (CD119−/−), and T cell α/β R knockout (TCR −/−) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CD118−/− (30), RNase L and PKR double knockout (RL/PKR −/−) mice, on a WT background (24, 31), are maintained at Dean McGee Eye Institute. Mice (ages 6–8 wks) were anesthetized by intraperitoneal injection with xylazine (2 mg/ml; 6.6 mg/kg) and ketamine (30 mg/ml; 100 mg/kg).
Cells
All cell culture reagents were obtained from Gibco (Grand Island, NY). Vero (African green monkey kidney) cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640, supplemented with 10% FBS, 2% antibiotic/antimycotic, and 0.2% gentamycin. Vero cells were plated in 96-well flat bottom plates (50,000 cells/well), and incubated at 37°C in an atmosphere of 5% CO2 and 95% humidity.
HSV-1 infection
Corneas were scarified 40 times with a 255/8-gauge needle. Next, tear films were blotted prior to topical inoculation with 10,000 PFUs HSV-1 (McKrae strain), prepared as described (32).
Adenoviral vector transduction
We utilized two replication defective adenoviral vectors (ΔE1-ΔE3), constructed as previously described (33, 34). Adenoviral vectors were propagated in E1-complementing E293 cells (American Type Culture Collection), in DMEM, supplemented with 5% FBS, 2% antibiotic/antimycotic, and 0.2% gentamycin. Cells were maintained at 37°C, 5% CO2, and 95% humidity. Mouse corneas were scarified as before, and tear films were blotted preceding topical application of 1x105 – 1x107 U control adenovirus vector (Ad: Null) or an adenovirus expressing the murine (mu) IFN-γ transgene.
Measurement of IFN-γ levels
At 48 h post-transduction (p.t.), corneas and TG were dissected. Each tissue sample was homogenized with a tissue miser (Fisher Scientific, Hampton, NH) in 500 μl T-PER tissue extraction reagent (Pierce, Rockford, IL), supplemented with a protease inhibitor cocktail (Calbiochem, San Diego, CA). Tissue homogenates were clarified by centrifugation at 10,000 g for 1–2 min. Levels of IFN-γ were determined by ELISA according to the manufacturer’s instructions (Quantikine immunoassay, R&D Systems, Minneapolis, MN).
Determination of HSV-1 titer
HSV-1 viral titers were examined at times post infection. Specifically, at d3 – 7 p.i., mice were euthanized and corneas and TG were dissected under sterile conditions. Tissues were frozen in 500 μl RPMI-1640 media, thawed, and homogenized using a tissue miser (Fisher Scientific, Hampton, NH). Tissue homogenates were then centrifuged at 10,000 g for 1–2 min. Clarified supernatants were serially diluted and incubated on Vero cell monolayers in 96-well microtiter plates for 60 min. Supernatants were subsequently discarded and replaced with a 100 μl overlay of RPMI-1640 containing 10% FBS, antibiotic/antimycotic, and 0.5% methylcellulose. Cultures were incubated at 37°C in 5% CO2 and 95% humidity for an additional 31 h. Amount of infectious virus has been reported as mean log PFU per cornea or TG.
Flow Cytometry
Mice were anesthetized and perfused with 20 ml PBS, pH 7.4. TG were collected and homogenized in complete DMEM medium, using a Dounce-type homogenizer. Homogenates were subsequently forced through a cell strainer (Becton Dickinson, Frankin Lakes, NJ). Corneas were collected and digested with 1 mg/ml collagenase type I (Sigma) for 90 min at 37°C. Single cell suspensions from cornea or TG were pelleted by centrifugation. Cells were incubated on ice for 15 min with 4 μl anti-mouse CD16/32 (BD Pharmingen, San Diego, CA) in 46 μl PBS-1% BSA. After the incubation, cells were centrifuged (300 g, 5 min) and resuspended in PBS-1% BSA containing 5% normal rat serum (Jackson Immuno Research Inc., West Grove, PA) for an additional 15 min on ice. Cells were then triple-stained in the dark at 4°C for 30 min in 50 μl PBS-1% BSA with BD Pharmingen antibodies: 2 μl FITC-conjugated anti-mouse CD3, 2 μl phycoerythrin-Cy5-conjugated anti-CD45 (clone 30-F11), and 2 μl PE labeled anti-mouse NK-1.1. Cells were then washed 3 times with PBS-1% BSA, pelleted each time by centrifugation (300 g, 5 min), and resuspended in PBS containing 1% paraformaldehyde. After overnight fixation at 4° C, cells were pelleted and resuspended in PBS-1% BSA. Before analysis, CountBright absolute counting beads (Invitrogen, Eugene, OR) were added (28,000/sample). Cell suspensions were gated on CD45high expressing cells, and the percentage of T (CD3+NK1.1−) and NK (CD3−NK1.1+) cells were determined at this gate setting. A second gate was used to count the number of beads that passed through during the sampling time. Samples were analyzed for 550 s; the absolute number of leukocytes (CD45high) in TG and cornea were determined by calculating: (n input beads/n beads counted per sample) X (n CD45high events). The absolute number of T and NK cells was then calculated: (n CD45high cells) X (% each lymphocyte population per sample). Isotypic control antibodies were utilized to establish background fluorescence levels.
Statistical analysis
Statistical analysis was performed using the GBSTAT program (Dynamic Microsystems, Silver Spring, MD). Specifically, Student’s t test (2 sample/separate variances) was used to determine significant differences between the Ad: Null and Ad: IFN-γ transduced groups.
Results
Ad:IFN-γ transduction prior to infection results in a dose-dependent reduction in infectious virus
Previously, 1x106 transducing units (TU) Ad:IFN-β was shown to be effective at enhancing the survival of HSV-1 infected mice (35). As a starting point to examine Ad: IFN-γ effectiveness, we also chose to utilize 1x106 TU adenoviral vector, as well as 1 log more and 1 log less than the effective dose seen in prior studies with Ad:IFN-β (35). With 1x105 TU Ad:IFN-γ, no IFN-γ was detected in cornea at 48 h post transduction (pt). By comparison, transduction of corneas with 1x106 or 1x107 TU Ad:IFN-γ resulted in detection of IFN-γ in 50% of corneas, ranging from 24–36 pg/cornea. We were unable to detect IFN-γ in Ad: Null transduced corneas with the same dose.
Since IFN-γ levels were successfully measured in the cornea following in situ transduction, WT mouse corneas were next transduced with 1x105–1x107 TU Ad:IFN-γ and assessed for anti-viral activity following inoculation of corneas with HSV-1. No differences in viral titers were found in mouse corneas or TG from animals transduced with 1x105 TU Ad:Null versus Ad:IFN-γ (Fig. 1). With 1x106 TU adenoviral vector, infectious virus levels were less in corneas and TG from Ad IFN-γ transduced mice, but the difference did not achieve significance (Fig. 1). With 1x107 TU, corneas and TG from mice transduced with Ad:IFN-γ possessed significantly less virus, compared with corneas and TG from Ad:Null transduced mice (Fig. 1).
Figure 1.
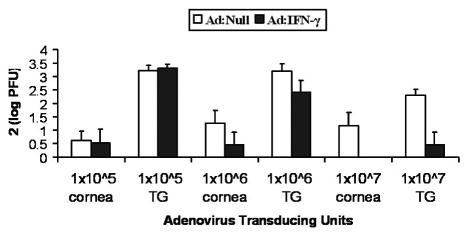
Dose dependent effectiveness of Ad: IFN-γ transduction. WT mouse corneas were transduced with 1x105 – 1x107 TU Ad: Null or Ad: IFN-γ, 24 h prior to infection with 10,000 PFU HSV-1. At d7 p.i. the amount of infectious virus was analyzed in each cornea and TG by plaque assay. Data represent collective log PFU/tissue x2 from a representative experiment (n = 4 samples/group), which was repeated 3 times.
In the absence of IFN-γ R, anti-viral action of Ad:IFN-γ transduction is lost
The IFN-γ receptor is composed of a ligand binding subunit, referred to as IFN γR1, IFNγRα, or CDW119 and a second receptor subunit, referred to as IFNγR2, IFNγRβ, or AF-1 (1). To verify the specificity of Ad:IFN-γ, mice deficient in IFN R1 (CD119 −/−) were transduced with 1x107 TU Ad:Null or Ad:IFN-γ, 24 h prior to infection with HSV-1. In the first experiment, we attempted to analyze infectious virus levels at d7 p.i., but CD119−/− mice died at d6 p.i. In two subsequent experiments, infectious virus levels were analyzed in corneas and TG at d5 p.i. Reduction in infectious virus was not found in corneas or TG from CD119−/− mice, transduced with Ad:IFN-γ versus Ad:Null (data not shown), suggesting that the Ad:IFN-γ elicited anti-viral effect is mediated through CD119.
The anti-viral effect of Ad: IFN –γ over time
Mouse corneas were transduced with Ad:Null or Ad:IFN-γ, 24 h prior to infection, and infectious virus levels were monitored at d3, d5, and d7 p.i. in the cornea and TG. At d3 p.i. there was no difference in viral titer in corneas from mice transduced with Ad:Null versus Ad:IFN-γ (Fig. 2). In cornea at d5 and d7 p.i. and in TG at d3-d7 p.i., infectious virus levels were reduced 1.5 to 2.5 logs in tissues from Ad:IFN-γ transduced mice, compared with Ad:Null control (Fig. 2). Notably, no virus was detected at d5 p.i. in 10/10 corneas from mice transduced with 1x107 TU Ad:IFN-γ (Fig. 2).
Figure 2.
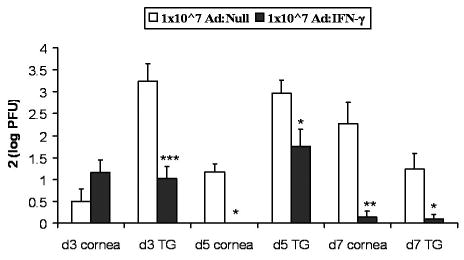
Time course analysis of Ad: IFN-γ efficacy. Following scarification, WT mouse corneas were transduced with Ad: Null or Ad: IFN-γ using 1x107 TU. Corneas were infected with 10,000 PFU HSV-1 24 h later. Viral titers were determined in corneas and TG by plaque assay at d3, d5, and d7 p.i. Data represent collective mean log PFU/tissue x2 from 2–3 separate experiments (n = 9–15 samples/time point and treatment). *p < 0.05, **p < 0.005, ***p < 0.001
Next, the prophylactic versus therapeutic application of Ad: IFN-γ was determined. Since, on occasion, Ad:Null transduced WT mice succumbed to infection prior to d7 p.i., tissue was harvested d5 p.i. in all transduced groups of mice. Transduction of mouse corneas with Ad:IFN-γ, 24 h prior (Figs. 2 and Figs. 4–6) and 72 h before infection (Fig 3), resulted in significant reduction of viral titers in TG and cornea, compared to virus levels in these tissues from Ad:Null transduced mice. When mice were transduced 24–72 h after infection, the anti-viral effect was no longer observed in the cornea (Fig. 3). However, transduction of corneas 24 h, but not 72 h after infection, suppressed viral replication in TG (Fig. 3). Ironically, transduction of Ad:IFN-γ at the time of infection didn’t impact HSV-1 replication in cornea or TG (Fig. 3).
Figure 4.
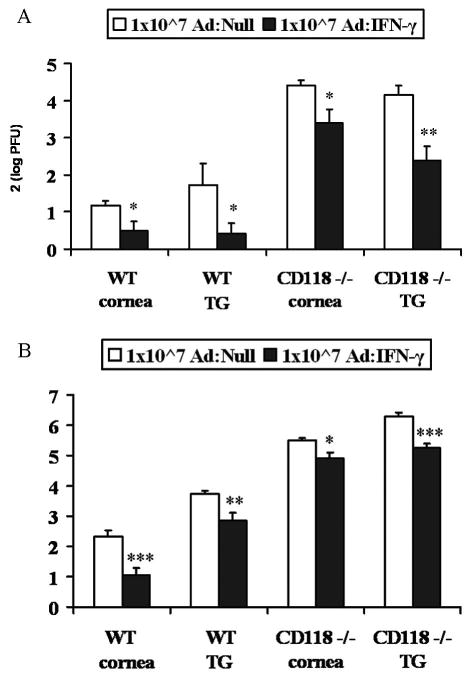
Efficacy of Ad: IFN-γ in CD118−/− mice. CD118−/− mouse corneas were scarified and transduced with 1x107 TU Ad: Null or Ad: IFN-γ, 24 hours prior to infection with 10,000 PFU HSV-1. Infectious virus levels were determined by plaque assay. A, Infectious virus levels at d3 p.i. are reported as cumulative mean log PFU/tissue +/− SEM x2 from 2 separate experiments (n = 8–10 samples/group). B, Infectious virus levels at d5 p.i. are reported as cumulative mean log PFU/tissue x2 +/− SEM from 3 separate experiments (n = 4–12 samples/group). *p<0.05, **p<0.005, ***p<0.001
Figure 6.
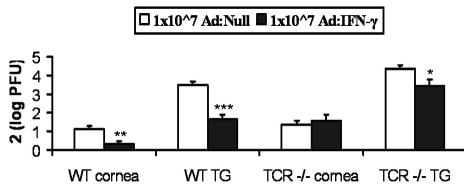
Ad: IFN-γ Protection in TCR −/− mice. WT and T Cell R deficient (TCR −/−) mouse corneas were scarified prior to transduction with 1x107 TU Ad: Null or Ad: IFN-γ. 24 h later corneas were infected with 10,000 PFU HSV-1. Infectious virus levels were analyzed by plaque assay in cornea and TG at d5 p.i. Data represent mean log PFU/tissue x2 +/− SEM from 3–4 experiments (n = 13–19 samples/group). *p< 0.05, **p < 0.005, ***p < 0.001
Figure 3.
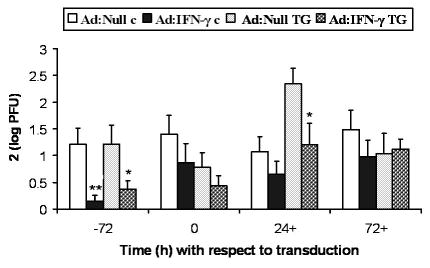
Kinetics of Ad: IFN-γ protection. Mouse corneas were scarified and transduced with 1x107 TU Ad: Null or Ad: IFN-γ 72 h prior (-72), at the same time (0), 24 h after (24+), or 72 hours after (72+) infection with 10,000 PFU HSV-1. Infectious HSV-1 levels in the cornea (c) and TG were analyzed by plaque assays at d5 p.i. Data represent collective mean log PFU/tissue x2 from 2 separate experiments (n = 10–12 samples/time point and treatment). *p< 0.05, **p< 0.005
The anti-viral effect of Ad: IFN-γ is not lost in the absence of CD118 (IFN α/β R)
To examine potential type I IFN involvement, the effectiveness of Ad:IFN-γ in mice devoid of one subunit of the type I IFN R (23, 30) was examined. In the absence of CD118, Ad: IFN-γ was capable of suppressing HSV-1 replication in both the cornea and TG at d3 (Fig. 4a) or d5 (Fig. 4b) p.i. It should be noted that 4/6 Ad:Null transduced CD118−/− mice succumbed to infection at d4 p.i., whereas none of the Ad:IFN-γ transduced mice died by d5 p.i. This observation reinforces the protective effect of Ad: IFN-γ even in the absence of a functional type I IFN R.
The anti-viral effect of Ad: IFN-γ is lost in mice devoid of functional OAS/PKR pathways
PKR and OAS are inducible following exposure to IFN-γ, and both have proven anti-viral activity (14). To determine if these pathways are involved in the Ad:IFN-γ inducible anti-viral effect in vivo, viral titers were compared in WT and RL/PKR deficient mice, transduced with Ad:Null versus Ad:IFN-γ, prior to infection with HSV-1. Consistent with previous results, Ad:IFN-γ transduced WT mice were found to possess significantly less HSV-1 in cornea and TG (Fig. 5). In contrast, the effect was lost in RL/PKR deficient mice (Fig. 5).
Figure 5.
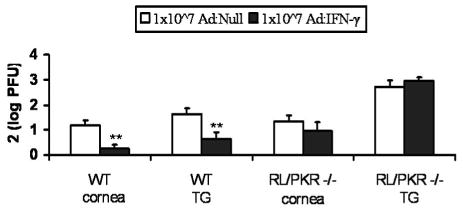
Ineffectiveness of Ad: IFN-γ transduction in RL/PKR −/− mice. WT and RNase L/PKR deficient (RL/PKR −/−) mouse corneas were transduced with 1x107 TU Ad: Null or Ad: IFN-γ 24 h prior to infection with 10,000 PFU HSV-1. At day 5 p.i., levels of infectious virus were determined by plaque assay for each cornea and TG. Data represent collective mean log PFU/tissue x2 from 2–3 separate experiments (n = 12–16 samples/group). **p < 0.01
Ad: IFN-γ partially protects against HSV-1 infection in TG from mice lacking α/β T Cell Receptor
Since activated CD4 and CD8 T cells are a rich source of IFN-γ, and both T cells and IFN-γ are found in the TG during acute ocular HSV-1 infection (25, 27, 28), we questioned whether Ad:IFN-γ could substitute for T cells in protecting mice against HSV-1 infection. Therefore, the anti-viral effect of Ad:IFN-γ was evaluated in α/β TCR −/− mice. The results show in mice lacking α/β TCR+ T cells, the anti-viral activity of Ad: IFN-γ was lost in the cornea and attenuated in the TG, compared to WT mice (Fig. 6).
T cell and NK cell recruitment to the TG of HSV-1 infected mice is diminished following Ad: IFN-γ transduction
Since Ad:IFN-γ antagonized HSV-1 replication, we reasoned that the reduced antigenic stimulus would equate to fewer leukocytes infiltrating the tissue. Therefore, leukocyte trafficking into TG and cornea was measured in mice transduced with Ad:IFN-γ. Within the TG, there were significantly fewer T cells in the Ad:IFN-γ transduced mice compared to TG from Ad:Null transduced mice (Fig 7a). Although not significant, a similar trend was found assessing NK cells with a reduction residing in the TG from Ad:IFN-γ transduced mice compared to the Ad:Null transduced animals (Fig 7a). The total leukocyte (CD45high) population infiltrating the TG of Ad:IFN-γ and Ad:Null were not significantly different (data not shown). In contrast, the total leukocyte infiltration in the cornea of Ad:Null transduced mice was significantly elevated in comparison to the cornea from Ad:IFN-γ transduced animals (Fig. 7b). Neither NK cell nor T cell populations residing in the cornea of mice transduced with Ad:Null or Ad:IFN-γ were found to be different (Fig. 7b).
Figure 7.
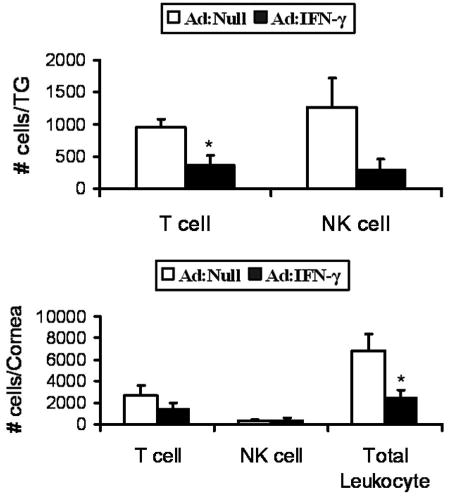
Recruitment of leukocytes to the cornea of Ad:Null and Ad:IFN-γ transduced mice in response to HSV-1. WT mouse corneas were scarified and transduced with 1x107 TU Ad: Null or Ad: IFN-γ. Corneas were infected with 10,000 PFU HSV-1 24 h post transduction. Mice were euthanized at day 5 post infection, and single cell suspensions were prepared from the TG (panel A) and cornea (panel B) and labeled for the detection of T cells (NK1.1−CD3+), NK cells (NK1.1+CD3−), and total leukocytes (CD45high) subsequently analyzed by flow cytometry. The results are shown as mean ± SEM from 2–3 separate experiments (n=3–6 samples/group). *p<0.05.
Discussion
HSV-1 infection of cornea triggers a potent inflammatory response that serves to eliminate virus. However, repeated inflammatory events lead to permanent corneal scarring and vision loss (36). Corneal replacement is not a viable solution because individuals with past incidence of herpetic keratitis are likely to have chronic recurrence (37, 38). Development of better therapeutic strategies to prevent vision loss from herpetic keratitis requires an understanding of protective endogenous anti-viral systems. Type I IFN and downstream effector protection against HSV-1 morbidity and mortality is well documented (16–18, 23, 24, 35, 39–45). Type II IFNs are equally important for clearing virus from cornea and TG (46, 47), but knowledge of the mechanism used by IFN-γ to control HSV-1 is deficient. Previously, type I and II IFN have been shown synergist control of HSV-1 (20–23, 48). Therefore, we sought to determine whether type I IFNs and downstream effectors, OAS and PKR, are required for IFN-γ inhibition of HSV-1. Since CD3+ T and NK cells contribute to viral clearance (11, 14, 19, 25–27, 41, 46, 49–65), we further examined involvement of α/β TCR and NK and CD3+ T cells. A replication defective adenoviral vector to deliver the IFN-γ transgene was chosen for the current study instead of treatment with rIFN-γ, since a continuous source of IFN-γ would be available. In addition, we reasoned this to be an improved approach since host cells profoundly affect post-translational processing, the three-dimensional structure of the glycoprotein, and its biological function (66–69).
In the present study, transduction of mouse corneas with Ad:IFN-γ was effective at reducing HSV-1 replication prior to or within 24 h after infection. However, transduction at the time of infection did not significantly impact viral replication. This result suggests that either IFN-γ protein was made at lower levels during infection, or the protein made was less functional. One explanation is that the virus caused a shut down of host cell protein synthesis, so IFN-γ was made at lower levels (70). Alternatively, alterations in cornea cell biology during infection caused IFN-γ to be less functional either by altering post-translational modifications or by a secondary effect on the conformation of the protein. While there are many possible explanations for viral interference with protein function, the activity of IFN-γ is known to be dependent on interactions with heparin sulfate. Specifically, binding to heparin sulfate influences both the activity and availability of IFN-γ (71). HSV-1 also uses heparin sulfate as its primary receptor (72), so availability of heparin sulfate for IFN-γ may be reduced at the time of infection.
In the present study, we found the levels of infectious virus were elevated in corneas and TG from type I IFN R deficient mice compared to WT mice. Yet, in the absence of CD118, the anti-viral efficacy of the Ad:IFN-γ was not lost. It was anticipated the effectiveness of Ad: IFN-γ would not require anti-viral pathways including OAS/PKR implicated in the inhibition of HSV-1 (16, 17, 39, 42, 43). In contrast to previous observations in vitro (15), effectiveness of IFN-γ transgene was lost in vivo in the absence of intact OAS/PKR signaling. Local levels of OAS and PKR transcripts were only modestly elevated (2 fold by real time RT-PCR) following Ad: IFN-γ transduction (not shown). Cumulatively, the results suggest the effect of IFN-γ on OAS/PKR is likely systemic. One possible interpretation is that the IFN-γ transgene reach distal locations and exert anti-viral activity, mediated by OAS/PKR, via organized immune tissue (18).
Along with activation of endogenous host anti-viral pathways, such as OAS/PKR, HSV-1 infection of cornea triggers a potent inflammatory response. Toll like receptors are activated, leading to production of CXC chemokines (73), also induced by IFN-γ, which act as chemoattractants for T and NK cells (72). While IFN-γ indirectly activates chemokines that function as chemoattractants for leukocytes, it could be argued that with IFN-γ treatment, infectious virus levels and viral antigen levels are lower, and therefore, there will be less immune cell infiltration. In mice lacking α/β TCR+ T cells, the efficacy of Ad:IFN-γ against HSV-1 replication was completely lost in cornea and partially lost in TG. We interpret these results to suggest that in TG the presence of IFN-γ can partially override the requirement for T cells. In the TG, fewer T cells were found in mice transduced with Ad:IFN-γ. But in cornea, there was no difference in T cell infiltrates in the mice transduced with Ad: Null or Ad: IFN-γ. Whether T-cells trafficking to corneas of Ad: IFN-γ versus Ad: Null transduced mice was functionally different was not analyzed. While the total number of T cells was not significantly different, the phenotype of CD4+ T cells could be different in Ad: IFN-γ transduced mice. We reason this may be the case from previous reports examining the immunosuppressive environment of the anterior ocular chamber. Under homeostatic conditions, the anterior chamber of the eye is has a specific deficiency of antigen-specific delayed hypersensitivity mediating T cells (DTH) - mediated by Th1 cells - and a shift towards a Th2 phenotype of CD4+ T cells (74). This situation would therefore be different than the normal immune environment in the TG, potentially explaining some of the differences observed in the effect of Ad: IFN-γ in cornea versus TG. Another factor that could contribute to differences in T cell phenotypes includes products of Th1 and Th2 cells cross regulate each other (74–76). Specifically, IFN-γ, a cytokine of Th1 cells, inhibits Th2 proliferation (75). It is difficult to separate out the players in ocular immune suppression in our experimental model because most antigens introduced into the anterior chamber prime/induce suppression of DTH (such as albumin, which would be present in the medium used to prepare our adenovirus and HSV-1 stocks), but herpes simplex introduction into the anterior chamber of C57BL/6 mice induces positive DTH (77), and local expression of IFN-γ also abolishes intraocular immune priviledge (78).
In conclusion, IFN-γ transgene delivery with an adenoviral vector potently reduced levels of infectious HSV-1 in cornea and TG during primary infection. The effectiveness of transgene delivery was dose-dependent and required IFN-γ R, intact anti-viral pathways (OAS/PKR) and the presence of α/β TCR+ T cells. A reduction in the total leukocyte infiltrate into the cornea of Ad:IFN-γ transduced mice following HSV-1 was also discovered. Excluding the T and NK cell population, the predominant infiltrating population of leukocytes includes PMNs and macrophages known to contribute in the inflammatory response through the production of soluble mediators including TNF-α and matrix metalloproteins that often lead to undesirable collateral damage (79). Therefore, the contribution of the Ad:IFN-γ transduction of corneal tissue may not only reside in a reduction in virus replication but also a reduction in the trafficking of PMNs and macrophages that would significantly contribute to impairment of the visual axis.
Acknowledgments
The authors gratefully acknowledge the technical assistance provided by Gabrielle Nguyen, Manoj Thapa, and Todd Wuest.
Abbreviations used in this paper
- PKR
double stranded RNA-dependent protein kinase
- OAS
oligoadenylate synthetase
- TG
trigeminal ganglia
- Ad: IFN-γ
adenoviral vector containing murine IFN-γ transgenes
- OAS/RL
reference to pathway for OAS and it’s downstream effector, RNase L
- WT
wild type
- CD118−/−
IFN α/β R deficient
- CD119−/−
interferon gamma R deficient
- TCR −/−
T cell α/β R knockout
- RL/PKR −/−
RNase L and PKR double knockout mice
- Ad: Null
adenoviral vector without IFN transgene
- mu
murine
- p.t.
post-transduction; -, time before infection
- 0
same time as infection
- +
time after infection
- log PFU
mean log PFU per tissue
- TU
transducing units
- DTH
delayed-type sensitivity
Footnotes
This work was supported by a Research to Prevent Blindness Stein Research Professorship (DJJC) and grants from the National Institutes of Health: AI053108 (DJJC), and core grant EY12190.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 2.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 3.Iordanov MS, Paranjape JM, Zhou A, Wong J, Williams BR, Meurs EF, Silverman RH, Magun BE. Activation of p38 mitogen-activated protein kinase and c-Jun NH(2)-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SB, Bablanian R, Esteban M. Regulated expression of the interferon-induced protein kinase p68 (PKR) by vaccinia virus recombinants inhibits the replication of vesicular stomatitis virus but not that of poliovirus. J Interferon Cytokine Res. 1996;16:1073–1078. doi: 10.1089/jir.1996.16.1073. [DOI] [PubMed] [Google Scholar]
- 5.Khabar KS, Dhalla M, Siddiqui Y, Zhou A, Al-Ahdal MN, Der SD, Silverman RH, Williams BR. Effect of deficiency of the double-stranded RNA-dependent protein kinase, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes. J Interferon Cytokine Res. 2000;20:653–659. doi: 10.1089/107999000414835. [DOI] [PubMed] [Google Scholar]
- 6.Theerasurakarn S, Ubol S. Apoptosis induction in brain during the fixed strain of rabies virus infection correlates with onset and severity of illness. J Neurovirol. 1998;4:407–414. doi: 10.3109/13550289809114539. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Guerra M, Rivas C, Esteban M. Inducible expression of the 2–5A synthetase/RNase L system results in inhibition of vaccinia virus replication. Virology. 1997;227:220–228. doi: 10.1006/viro.1996.8294. [DOI] [PubMed] [Google Scholar]
- 8.Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlovic J, Arzet HA, Hefti HP, Frese M, Rost D, Ernst B, Kolb E, Staeheli P, Haller O. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J Virol. 1995;69:4506–4510. doi: 10.1128/jvi.69.7.4506-4510.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staeheli P, Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991;65:4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komatsu T, Bi Z, Reiss CS. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 12.Lowenstein CJ, Hill SL, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose NR, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detels R, Brody JA, McNew J, Edgar AH. Further epidemiological studies of subacute sclerosing panencephalitis. Lancet. 1973;2:11–14. doi: 10.1016/s0140-6736(73)91946-6. [DOI] [PubMed] [Google Scholar]
- 14.Chesler DA, Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002;13:441–454. doi: 10.1016/s1359-6101(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 15.Austin BA, Halford W, Silverman RH, Williams BR, Carr DJ. OAS and PKR are not required for the antiviral effect of Ad:IFN-gamma against acute HSV-1 in primary trigeminal ganglia cultures. J Interferon Cytokine Res. 2006;26:220–225. doi: 10.1089/jir.2006.26.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Khatib K, Williams BR, Silverman RH, Halford W, Carr DJ. Distinctive roles for 2′,5′-oligoadenylate synthetases and double-stranded RNA-dependent protein kinase R in the in vivo antiviral effect of an adenoviral vector expressing murine IFN-beta. J Immunol. 2004;172:5638–5647. doi: 10.4049/jimmunol.172.9.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-khatib K, Williams BR, Silverman RH, Halford W, Carr DJ. The murine double-stranded RNA-dependent protein kinase PKR and the murine 2′,5′-oligoadenylate synthetase-dependent RNase L are required for IFN-beta-mediated resistance against herpes simplex virus type 1 in primary trigeminal ganglion culture. Virology. 2003;313:126–135. doi: 10.1016/s0042-6822(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 18.Noisakran S, Carr DJ. Topical application of the cornea post-infection with plasmid DNA encoding interferon-alpha1 but not recombinant interferon-alphaA reduces herpes simplex virus type 1-induced mortality in mice. J Neuroimmunol. 2001;121:49–58. doi: 10.1016/s0165-5728(01)00442-8. [DOI] [PubMed] [Google Scholar]
- 19.Vollstedt S, Arnold S, Schwerdel C, Franchini M, Alber G, Di Santo JP, Ackermann M, Suter M. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J Virol. 2004;78:3846–3850. doi: 10.1128/JVI.78.8.3846-3850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce AT, DeSalvo J, Foster TP, Kosinski A, Weller SK, Halford WP. Beta interferon and gamma interferon synergize to block viral DNA and virion synthesis in herpes simplex virus-infected cells. J Gen Virol. 2005;86:2421–2432. doi: 10.1099/vir.0.80979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halford WP, Halford KJ, Pierce AT. Mathematical analysis demonstrates that interferons-beta and -gamma interact in a multiplicative manner to disrupt herpes simplex virus replication. J Theor Biol. 2005;234:439–454. doi: 10.1016/j.jtbi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Neumann-Haefelin D, Sundmacher R, Frey H, Merk W. Recombinant HuIFN-gamma prevents herpes simplex keratitis in African green monkeys: demonstration of synergism with recombinant HuIFN-alpha 2. Med Microbiol Immunol (Berl) 1985;174:81–86. doi: 10.1007/BF02123229. [DOI] [PubMed] [Google Scholar]
- 23.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 24.Zhou A, Paranjape J, Brown TL, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman RH. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. Embo J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill TJ. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 26.Manickan E, Rouse BT. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J Virol. 1995;69:8178–8179. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimeld C, Whiteland JL, Williams NA, Easty DL, Hill TJ. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J Gen Virol. 1997;78:3317–3325. doi: 10.1099/0022-1317-78-12-3317. [DOI] [PubMed] [Google Scholar]
- 29.Feduchi E, Alonso MA, Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989;63:1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garvey TL, Dyer KD, Ellis JA, Bonville CA, Foster B, Prussin C, Easton AJ, Domachowske JB, Rosenberg HF. Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J Immunol. 2005;175:4735–4744. doi: 10.4049/jimmunol.175.7.4735. [DOI] [PubMed] [Google Scholar]
- 31.Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams BR, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. Embo J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halford WP, Gebhardt BM, Carr DJ. Mechanisms of herpes simplex virus type 1 reactivation. J Virol. 1996;70:5051–5060. doi: 10.1128/jvi.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolls J, Peppel K, Silva M, Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei D, Lancaster JR, Jr, Joshi MS, Nelson S, Stoltz D, Bagby GJ, Odom G, Shellito JE, Kolls JK. Activation of alveolar macrophages and lung host defenses using transfer of the interferon-gamma gene. Am J Physiol. 1997;272:L852–L859. doi: 10.1152/ajplung.1997.272.5.L852. [DOI] [PubMed] [Google Scholar]
- 35.Al-Khatib K, Williams BR, Silverman RH, Halford W, Carr DJ. Dichotomy between survival and lytic gene expression in RNase L- and PKR-deficient mice transduced with an adenoviral vector expressing murine IFN-beta following ocular HSV-1 infection. Exp Eye Res. 2005;80:167–173. doi: 10.1016/j.exer.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell WJ, Gressens P, Martin JR, DeSanto R. Herpes simplex virus type 1 DNA persistence, progressive disease and transgenic immediate early gene promoter activity in chronic corneal infections in mice. J Gen Virol. 1994;75:1201–1210. doi: 10.1099/0022-1317-75-6-1201. [DOI] [PubMed] [Google Scholar]
- 38.Nesburn AB. Recurrence in ocular herpes simplex infection. Int Ophthalmol Clin. 1975;15:101–110. doi: 10.1097/00004397-197501540-00009. [DOI] [PubMed] [Google Scholar]
- 39.Carr DJ, Al-khatib K, James CM, Silverman R. Interferon-beta suppresses herpes simplex virus type 1 replication in trigeminal ganglion cells through an RNase L-dependent pathway. J Neuroimmunol. 2003;141:40–46. doi: 10.1016/s0165-5728(03)00216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 42.Al-Khatib K, Williams BR, Silverman RH, Halford WP, Carr DJ. Absence of PKR attenuates the anti-HSV-1 activity of an adenoviral vector expressing murine IFN-beta. J Interferon Cytokine Res. 2002;22:861–871. doi: 10.1089/107999002760274872. [DOI] [PubMed] [Google Scholar]
- 43.Austin BA, James C, Silverman RH, Carr DJ. Critical role for the oligoadenylate synthetase/RNase L pathway in response to IFN-beta during acute ocular herpes simplex virus type 1 infection. J Immunol. 2005;175:1100–1106. doi: 10.4049/jimmunol.175.2.1100. [DOI] [PubMed] [Google Scholar]
- 44.Carr DJ, Veress LA, Noisakran S, Campbell IL. Astrocyte-targeted expression of IFN-alpha1 protects mice from acute ocular herpes simplex virus type 1 infection. J Immunol. 1998;161:4859–4865. [PubMed] [Google Scholar]
- 45.Kimura T, Murata Y, Yamaguchi T, Okumura K. [Immunopathological analysis of T and non-T cell involvement in corneal inflammation caused by HSV type-1 infection] Nippon Ganka Gakkai Zasshi. 1989;93:124–131. [PubMed] [Google Scholar]
- 46.Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 47.He J, Ichimura H, Iida T, Minami M, Kobayashi K, Kita M, Sotozono C, Tagawa YI, Iwakura Y, Imanishi J. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J Interferon Cytokine Res. 1999;19:609–615. doi: 10.1089/107999099313749. [DOI] [PubMed] [Google Scholar]
- 48.Sainz B, Jr, Halford WP. Alpha/Beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J Virol. 2002;76:11541–11550. doi: 10.1128/JVI.76.22.11541-11550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heiligenhaus A, Bauer D, Zheng M, Mrzyk S, Steuhl KP. CD4+ T-cell type 1 and type 2 cytokines in the HSV-1 infected cornea. Graefes Arch Clin Exp Ophthalmol. 1999;237:399–406. doi: 10.1007/s004170050251. [DOI] [PubMed] [Google Scholar]
- 50.Geiger KD, Nash TC, Sawyer S, Krahl T, Patstone G, Reed JC, Krajewski S, Dalton D, Buchmeier MJ, Sarvetnick N. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 51.Banerjee K, Biswas PS, Kumaraguru U, Schoenberger SP, Rouse BT. Protective and pathological roles of virus-specific and bystander CD8+ T cells in herpetic stromal keratitis. J Immunol. 2004;173:7575–7583. doi: 10.4049/jimmunol.173.12.7575. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005;77:24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee K, Deshpande S, Zheng M, Kumaraguru U, Schoenberger SP, Rouse BT. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol. 2002;219:108–118. doi: 10.1016/s0008-8749(02)00601-9. [DOI] [PubMed] [Google Scholar]
- 54.Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 55.Brandt CR, Salkowski CA. Activation of NK cells in mice following corneal infection with herpes simplex virus type-1. Invest Ophthalmol Vis Sci. 1992;33:113–120. [PubMed] [Google Scholar]
- 56.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carr DJ. Increased levels of IFN-gamma in the trigeminal ganglion correlate with protection against HSV-1-induced encephalitis following subcutaneous administration with androstenediol. J Neuroimmunol. 1998;89:160–167. doi: 10.1016/s0165-5728(98)00129-5. [DOI] [PubMed] [Google Scholar]
- 58.Costa-Pereira AP, Williams TM, Strobl B, Watling D, Briscoe J, Kerr IM. The antiviral response to gamma interferon. J Virol. 2002;76:9060–9068. doi: 10.1128/JVI.76.18.9060-9068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glenn JA, Sonceau JB, Wynder HJ, Thomas WE. Histochemical evidence for microglia-like macrophages in the rat trigeminal ganglion. J Anat. 1993;183:475–481. [PMC free article] [PubMed] [Google Scholar]
- 61.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia LK, Zhang JS, Chen XL, Wang AY. [Cytokine expression in murine cornea tissue during recurrent herpetic stromal keratitis] Zhonghua Yan Ke Za Zhi. 2005;41:403–408. [PubMed] [Google Scholar]
- 64.Seo SK, Gebhardt BM, Lim HY, Kang SW, Higaki S, Varnell ED, Hill JM, Kaufman HE, Kwon BS. Murine keratocytes function as antigen-presenting cells. Eur J Immunol. 2001;31:3318–3328. doi: 10.1002/1521-4141(200111)31:11<3318::aid-immu3318>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Hendricks RL. An immunologist's view of herpes simplex keratitis: Thygeson Lecture 1996, presented at the Ocular Microbiology and Immunology Group meeting, October 26, 1996. Cornea. 1997;16:503–506. [PubMed] [Google Scholar]
- 66.James DC, Freedman RB, Hoare M, Ogonah OW, Rooney BC, Larionov OA, Dobrovolsky VN, Lagutin OV, Jenkins N. N-glycosylation of recombinant human interferon-gamma produced in different animal expression systems. Biotechnology (N Y) 1995;13:592–596. doi: 10.1038/nbt0695-592. [DOI] [PubMed] [Google Scholar]
- 67.James DC, Goldman MH, Hoare M, Jenkins N, Oliver RW, Green BN, Freedman RB. Posttranslational processing of recombinant human interferon-gamma in animal expression systems. Protein Sci. 1996;5:331–340. doi: 10.1002/pro.5560050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hooker AD, Green NH, Baines AJ, Bull AT, Jenkins N, Strange PG, James DC. Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotechnol Bioeng. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 69.Cencic A, LeFevre F, Koren S, La Bonnardiere C. Tetracycline-controlled expression of glycosylated porcine interferon-gamma in mammalian cells. Anim Biotechnol. 1999;10:63–79. doi: 10.1080/10495399909525922. [DOI] [PubMed] [Google Scholar]
- 70.Laurent AM, Madjar JJ, Greco A. Translational control of viral and host protein synthesis during the course of herpes simplex virus type 1 infection: evidence that initiation of translation is the limiting step. J Gen Virol. 1998;79:2765–2775. doi: 10.1099/0022-1317-79-11-2765. [DOI] [PubMed] [Google Scholar]
- 71.Lortat-Jacob H, Esterre P, Grimaud JA. Interferon-gamma, an anti-fibrogenic cytokine which binds to heparan sulfate. Pathol Res Pract. 1994;190:920–922. doi: 10.1016/S0344-0338(11)80996-9. [DOI] [PubMed] [Google Scholar]
- 72.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wuest T, Austin BA, Uematsu S, Thapa M, Akira S, Carr DJ. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol. 2006;179:46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li XY, D'Orazio LT, Niederkorn JY. Role of Th1 and Th2 cells in anterior chamber-associated immune deviation. Immunology. 1996;89:34–40. doi: 10.1046/j.1365-2567.1996.d01-714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garside P, Mowat AM. Polarization of Th-cell responses: a phylogenetic consequence of nonspecific immune defence? Immunol Today. 1995;16:220–223. doi: 10.1016/0167-5699(95)80162-6. [DOI] [PubMed] [Google Scholar]
- 76.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 77.Kielty D, Cousins SW, Atherton SS. HSV-1 retinitis and delayed hypersensitivity in DBA/2 and C57BL/6 mice. Invest Ophthalmol Vis Sci. 1987;28(12):1994–1999. [PubMed] [Google Scholar]
- 78.Geiger K, Sarvetnick N. Local production of IFN-gamma abrogates the intraocular immune privilege in transgenic mice and prevents the induction of ACAID. J Immunol. 1994;153:5239–5246. [PubMed] [Google Scholar]
- 79.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathological disease. Herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]


