Abstract
Francisella tularensis (Ft) is a Gram-negative bacterium and the causative agent of tularemia. It is well established that this organism replicates inside macrophages, but we are only beginning to understand this interface at the molecular level. Herein, we compared directly the ability of Ft subspecies holarctica live-vaccine strain to infect freshly isolated human peripheral blood monocytes, monocyte-derived macrophages (MDM), and cells of the murine macrophage cell line J774A.1 (J774). We now show that unopsonized bacteria infected human MDM fivefold more efficiently than monocytes or J774 cells in standard media. Moreover, enhanced infection of MDM was mediated, in part, by the macrophage mannose receptor (MR). Forming Ft phagosomes accumulated MR, and infection was inhibited by MR-blocking antibody or soluble mannan but not by the dectin-1 ligand laminarin. Up-regulation of MR in MDM (by exposure to interleukin-4) increased Ft phagocytosis, as did expression of MR in J774 cells. Conversely, opsonized Ft were ingested readily by monocytes and MDM. Medium supplementation with 2.5% fresh autologous serum was sufficient to confer opsonophagocytosis and CD11b accumulated in the membrane at sites of Ft engulfment. Infection of monocytes by opsonized Ft was nearly ablated by complement receptor 3 (CR3) blockade. Conversely, MDM used MR and CD11b/CD18 to ingest opsonized organisms. Altogether, our data demonstrate differential infection of mononuclear phagocytes by Ft and define distinct roles for MR and CR3 in phagocytosis.
Keywords: phagocytosis, complement, live-vaccine strain, MDM, tularemia, lectinophagocytosis
INTRODUCTION
Francisella tularensis (Ft) is a small, pleomorphic, Gram-negative bacterium and the causative agent of tularemia. There are four subspecies (subsp.) of this organism, but only two, Ft subsp. tularensis and Ft subsp. holarctica, cause disease in humans [1, 2]. Cutaneous, ulceroglandular infection with Ft occurs following direct contact with infected animals or insect vectors and is characterized by local lymphadenopathy and suppuration. Alternatively, inhalation of this organism causes a severe, systemic infection with fevers, chills, malaise, and pneumonia. As inhalation of as few as 10 organisms can cause disease, and 30–60% of untreated infections are fatal, Ft has been classified as a Category A select agent [1].
A key element of pathogenesis is the ability of Ft to evade killing by macrophages [3]. Although we know little about this infection at the molecular level, published data indicate that Ft phagosomes resist fusion with lysosomes [4], and late in infection, bacteria escape from the phagosome and replicate in the macrophage cytosol [5–7]. At the same time, the receptors that mediate phagocytosis of unopsonized Ft are unknown. This issue is of interest, as many studies of Ft-macrophage interactions have used the murine macrophage-like cell line J774A.1 (J774) [5, 8–11]. In this system, multiplicities of infection (MOI) of 100:1 or more are required to achieve infection [5, 12–14], which contrasts markedly with the ability of a few as 10 organisms to cause severe, fatal disease in mice and humans [1, 15]. Recent data suggest that freshly isolated human peripheral blood monocytes are also difficult to infect with Ft [6, 16]. The molecular basis for this low infection efficiency is unknown, and receptors that confer binding and phagocytosis of Ft have not been defined. Understanding how Ft gains entry into macrophages is important, as mechanism of entry can alter microbe fate [17].
In this study, we compared directly the ability of Ft subsp. holarctica live-vaccine strain (LVS) to infect J774 cells, human monocytes, and monocyte-derived macrophages (MDM). We now show that MDM are significantly more susceptible to infection with unopsonized Ft than blood monocytes or macrophage-like cell lines. Moreover, we demonstrate for the first time a specific role for the macrophage mannose receptor (MR) in phagocytosis of unopsonized Ft and define distinct roles for MR and CD11b/CD18 in uptake of opsonized bacteria.
MATERIALS AND METHODS
Materials
Endotoxin-free Dulbecco’s modified Eagle’s medium (DMEM), HEPES-buffered RPMI 1640 (hereafter, referred to as RPMI), L-glutamine, and phosphate-buffered saline (PBS) were from BioWhittaker/Cambrex (Walkersville, MD), and fetal bovine serum (FBS) was from HyClone (Logan, UT). Anti-Ft antiserum and monoclonal antibody (mAb) to CD18 (L130) were from BD Biosciences (San Diego, CA). Anti-Ft lipopolysaccharide (LPS; FB11) mAb was from QED Bioscience (San Diego, CA). Anti-CD11b mAb (M1/70.15.11.5.2), mouse anti-human lysosome-associated membrane protein-1 (lamp-1) mAb (H4A3), and rat anti-mouse lamp-1 mAb (1D4B) were from the Developmental Studies Hybridoma Bank at the University of Iowa (Iowa City). Anti-MR (MR5D3) mAb was from Serotec (Oxford, UK). Anti-CD11a (25.3.1) and anti-CD11b (Bear1) mAb were from Immunotech (Marseille, France) and Biodesign (Saco, ME), respectively. Antibody to mannose-binding lectin (MBL; D8.18) was from Cell Sciences (Canton, MA). Rabbit anti-Helicobacter pylori polyclonal antibodies were from Accurate Chemical and Scientific Corp. (Hicksville, NY). Fluorescein isothiocyanate (FITC)- and rhodamine-conjugated immunoglobulin G (IgG) F(ab′)2 secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Recombinant human interleukin-4 (rhIL-4) was from R&D Systems (Minneapolis, MN). Other reagents were from Sigma-Aldrich (St. Louis, MO).
Cultivation of bacteria
Ft subsp. holarctica LVS was obtained from Dr. Michael Apicella (University of Iowa). Bacteria were inoculated onto sheep blood cysteine heart agar from frozen stocks and incubated for 48 h in a humidified incubator at 37°C. Bacterial colonies were harvested from plates, washed twice in PBS, and quantified by measuring the absorbance at 600 nm. H. pylori strain 11637 was cultivated on pH 6 sheep blood agar plates (37°C, 5% O2) under microaerophilic conditions as we described [18]. Staphylococcus aureus strain ALC 1435 [19] was grown overnight with shaking at 37°C in tryptic soy broth as described previously [20].
Mononuclear cell isolation and culture
Heparinized venous blood was obtained from healthy adult volunteers using a protocol approved by the Institutional Review Board for Human Subjects at the University of Iowa, and all participants provided informed consent. Mononuclear cells were isolated by centrifugation on Ficoll-Hypaque, washed twice in RPMI, resuspended in RPMI + 20% autologous serum (AS) at a concentration of 2 × 106/ml, and differentiated into macrophages by incubation in Teflon jars for 5–7 days at 37°C [21]. Where indicated, MDM were treated with 1000 IU/ml rhIL-4 on Day 5 and incubated an additional 48 h to induce alternative activation [22]. Monocytes were obtained by plating freshly isolated peripheral blood mononuclear cells onto chamberslides (Nunc, Rochester, NY). After 2 h at 37°C, monocyte monolayers were washed twice to remove nonadherent lymphocytes. J774 cells (obtained from the American Type Culture Collection, Manassas, VA) and MR-positive J774-E clone cells [23] (obtained from Philip D. Stahl, Washington University, St. Louis, MO) were maintained in DMEM containing 10% heat-inactivated (HI) FBS and 2 mM L-glutamine.
Phagocytosis
J774 cells, monocytes, or MDM were plated onto chamberslides (104 cells/well) in tissue-culture medium and allowed to adhere for 2 h at 37°C. Thereafter, MDM and monocytes were washed twice with PBS to remove nonadherent lymphocytes, and all samples were infected with LVS in fresh medium at a MOI of 20:1 or H. pylori at a MOI of 10:1. After 1 h at 37°C, media were removed, cells were washed twice with PBS to remove any uningested, noncell-associated bacteria, and samples were processed for microscopy as described below. For experiments assessing receptor recruitment to forming phagosomes, infections were synchronized as we described previously [24]. Briefly, Ft were bound to adherent MDM or monocytes by a 4-min, 12°C spin at 600 g, and phagocytosis was initiated by rapid transfer to a 37°C incubator. After 5 min at 37°C, samples were fixed and processed as described below.
Receptor blockade
To block certain lectin receptors, MDM were incubated with 0.1–3.0 mg/ml mannan, 0.1–3.0 mg/ml laminarin, or 10 μg/ml anti-MR blocking antibody in RPMI containing 2.5% AS or 10% HI-FBS for 15 min at 37°C prior to addition of bacteria. To block complement receptors (CRs), phagocytes were incubated with anti-CD18, anti-CD11b, anti-CD11a, or anti-CD18 and anti-CD11b blocking antibodies at 5–25 μg/ml in RPMI containing 2.5% AS or 10% HI-FBS at 37°C for 5 min prior to infection.
Microscopy
Infected phagocytes were processed for microscopy using our established methods [24] with minor modifications. Cells were fixed with 10% formalin, permeabilized using –20°C methanol-acetone (1:1), and then blocked overnight in PBS supplemented with 10% horse serum and 1 mg/ml bovine serum albumin (BSA). Fixed and permeabilized cells were double-stained to detect Ft and CD11b (M1/70.15.11.5.2), MR, or lamp-1, respectively. In all cases, secondary antibodies were conjugated to FITC or rhodamine. Coverslips were mounted to glass slides in gelvatol, and samples were examined using an LSM-510 confocal microscope or an Axioplan2 fluorescence microscope (both from Carl Zeiss, Inc., Thornwood, NY).
To quantify directly internalization of cell-associated Ft and H. pylori, the differential staining method of Heesemann and Laufs [25] was used as we described previously [26]. Briefly, mononuclear phagocytes infected with LVS or H. pylori for 60 min at 37°C were chilled on ice, washed with cold PBS, and then incubated on ice for 30–60 min in PBS containing anti-LVS or anti-H. pylori antibodies. After additional PBS washes, cells were fixed and permeabilized using methanol (10 min, 25°C) and then rehydrated in PBS supplemented with 5 mg/ml BSA. Extracellular bacteria were stained red by incubating cells in rhodamine-conjugated secondary F(ab′)2 IgG (30 min, 25°C). Fixed and permeabilized cells were subjected to another round of staining with primary antibody followed by secondary antibody conjugated to FITC. All assays were performed in triplicate, and samples were examined using phase and fluorescence optics. In each case, the number of green (total cell-associated) and red (bound, extracellular) bacteria was counted, and the number of intracellular bacteria was determined by subtraction. By this assay, 85–95% of all cell-associated bacteria were phagocytosed, and engulfment was ablated (<2% ingested organisms) by the F-actin destabilizing agent latrunculin B or by infection of cells at 4°C (data not shown). Data are presented as phagocytic indices (intracellular bacteria per 100 cells).
Mannose binding lectin
Ft LVS and S. aureus were incubated at a concentration of 107/ml in RPMI containing 0, 2.5, or 10% AS for 30 min at 37°C. After two washes with PBS, samples were solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Ten million cell equivalents from each sample were resolved by 8% SDS-PAGE and transferred to polyvinylidene difluoride membranes. MBL and Ft LPS were detected by immunoblotting using secondary antibody conjugated to horseradish peroxidase [26]. Bands were developed using enhanced chemiluminescence reagents (Pierce, Rockford, IL).
Statistics
Data were evaluated using one-way ANOVA or by the unpaired Student’s t-test (with the f-test to assess variance). In all cases, P < 0.05 was considered significant.
RESULTS
Differential infection of mononuclear phagocytes by Ft LVS
In our first series of experiments, we compared directly the ability of unopsonized Ft LVS to infect human monocytes, MDM, and murine J774 cells in standard tissue-culture media. As shown in Figure 1, infection of J774 cells with unopsonized Ft LVS was inefficient, and a MOI of 20:1 resulted in only 1.7 ± 0.8 bacteria/100 macrophages. Phagocytosis of Ft by blood monocytes was also a rare event (1.2±0.6 Ft/100 cells). Conversely, infection of MDM was significantly more efficient, and after 1 h at 37°C, these cells contained fivefold more bacteria than monocytes or J774 cells (7.7±1.8 Ft/100 MDM; Fig. 1A). In all cases, differential staining and colocalization with lamp-1 demonstrated that 85–95% of phagocyte-associated bacteria were engulfed (not illustrated). These data indicate that unopsonized Ft infects MDM more readily than blood monocytes or J774 cells. By contrast, all three cell types ingested large numbers of H. pylori (Fig. 1B), another Gram-negative pathogen, which resists killing by macrophages [18].
Fig. 1.
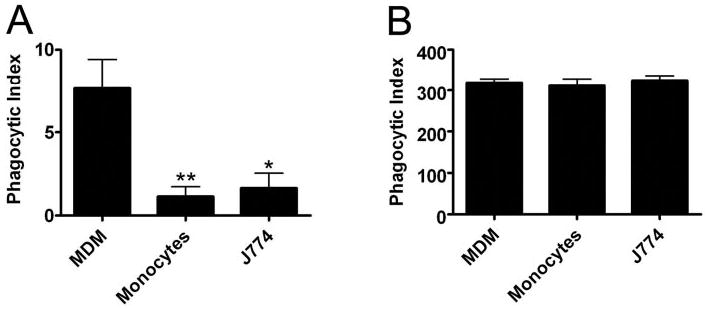
Differential infection of mononuclear phagocytes by Ft LVS. LVS at a MOI of 20:1 (A) or H. pylori at a MOI of 10:1 (B) was added to J774 cells in DMEM + 10% HI-FBS or to monocytes and MDM in RPMI + 10% HI-FBS. Phagocytic indices were scored after 1 h at 37°C. Data are the average ± SEM of three independent experiments performed in triplicate. *, P < 0.05; **, P < 0.01, versus MDM.
Role for the macrophage MR in Ft phagocytosis
The data shown above underscore the fact that not all mono-nuclear phagocytes are equivalent and further suggest that Ft may engage preferentially receptors that are present on MDM but not on monocytes and J774 cells. Of interest in this regard is MR. MRs are abundant on primary macrophages, including MDM, but are neither synthesized by nor present on blood monocytes and J774 cells [27]. This receptor recognizes a variety of carbohydrate ligands on microbial surfaces, and previous studies have defined a role for MR in phagocytosis of Mycobacterium tuberculosis, Pneumocystis carinii, and Leishmania donovani [28]. Accordingly, we assessed the role of MR in phagocytosis of unopsonized Ft LVS. Addition of soluble yeast mannan to culture medium is a well-established mechanism used to block MR binding via competitive inhibition [29]. Using this approach, we found that mannan inhibited MDM uptake of Ft in a dose-dependent manner (Fig. 2A). Phagocytosis was impaired significantly in medium containing 0.1 mg/ml or 1.0 mg/ml mannan (50% and 36% of control, respectively, P<0.01; Fig. 2A). Higher concentrations of mannan (3 mg/ml) had no additional effect (not illustrated). Specificity for MR is indicated by the fact that laminarin, a competitive inhibitor of the β-glucan receptor dectin-1 [30], had no effect on Ft uptake (Fig. 2A), and neither mannan nor laminarin (1–3 mg/ml) prevented phagocytosis of H. pylori (107±15% and 94±3% of control, respectively).
Fig. 2.
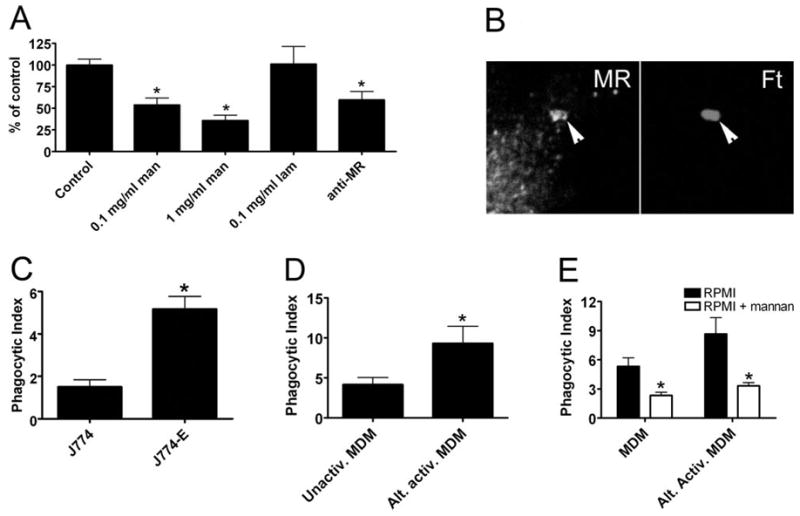
MRs mediate phagocytosis of unopsonized Ft. (A) MDM in RPMI + 10% HI-FBS were preincubated with the indicated concentrations of mannan (man), laminarin (lam), or 10 μg/ml anti-MR antibody prior to addition of Ft at a MOI of 20:1. After 1 h at 37°C, phagocytosis was quantified as in Figure 1. Data indicate the mean ± SEM from at least three independent experiments for each condition and are normalized to the no-antibody control (9±2 Ft/100 MDM). *, P < 0.01, versus control. (B) Confocal sections show enrichment of MR on forming (5 min) LVS phagosomes (arrowheads). (C) J774 and J774-E cells in DMEM + 10% HI-FBS were infected with LVS (MOI 20:1), and phagocytosis was quantified after 1 h at 37°C. Data are the mean ± SD of triplicate samples from a representative experiment. *, P < 0.01. (D) MDM were left untreated or exposed to IL-4 for 48 h to induce alternative activation (Alt. activ.) prior to infection with LVS at a MOI of 20:1 for 60 min. Data are the mean ± SEM from three independent experiments performed in triplicate. *, P < 0.05. (E) MDM and alternatively activated MDM were incubated in RPMI containing 10% HI-FBS in the presence and absence of 0.1 mg/ml mannan prior to addition of Ft at a MOI of 20:1, and phagocytosis was quantified after 1 h at 37°C. Data are the mean ± SD of triplicate samples from one experiment representative of three independent determinations. *, P < 0.05, versus no mannan control.
Although MR was the first mannan-sensitive receptor identified on macrophages, it is now clear that MR is part of a larger family of lectin receptors with similar ligand specificity [31, 32]. Therefore, we used a receptor-blocking antibody [33] to test directly the role of MR in Ft engulfment. As shown in Figure 2A, direct blockade of MR significantly impaired Ft phagocytosis (P<0.01). Moreover, mannan and MR-blocking antibody inhibited Ft uptake to a similar extent. In good agreement with these data, confocal imaging demonstrated that MR was recruited to, and accumulated on, forming Ft phagosomes (Fig. 2B). We also determined whether expression of MR in J774 cells was sufficient to enhance Ft uptake. Indeed, MR-positive J774-E cells [23] ingested threefold more Ft than MR-negative J774 (Fig. 2C, P<0.01), and this enhanced infection was ablated by 0.1 mg/ml mannan (33±15% of no mannan J774-E control, n=3). Altogether, these data define for the first time a role for MR in phagocytosis of unopsonized Ft LVS.
Macrophages treated with IL-4 undergo a phenotypic shift and achieve a state of alternative activation, which favors humoral immunity and tissue repair and is accompanied by changes in expression of many receptors including MR [34, 35]. As alternatively activated macrophages up-regulate MR, we predicted that these cells may exhibit enhanced phagocytosis of Ft. To test this hypothesis, we treated MDM with rhIL-4 prior to Ft infection. As shown in Figure 2D, IL-4-treated MDM ingested twice as many bacteria as resting MDM. The extent to which enhanced uptake of Ft is mediated by MR alone or MR in combination with other receptors remains to be determined. Nevertheless, it is noteworthy that infection of alternatively activated MDM was impaired significantly in the presence of 0.1 mg/ml mannan and that resting and IL-4-treated MDM ingested similar numbers of Ft by mannan-insensitive mechanisms (Fig. 2E). Collectively, these data suggest that alternatively activated macrophages are highly susceptible to Ft infection.
Serum complement enhances phagocytosis of Ft LVS by monocytes and MDM
As monocytes were refractory to infection with unopsonized Ft, we assessed the effect of fresh serum on phagocytosis. As shown in Figure 3B, supplementation of RPMI with 2.5% fresh AS supported robust infection of monocytes, and the number of bacteria engulfed increased 35-fold relative to monocytes in standard media. Fresh AS had a significant (but less marked) effect on infection of MDM (Fig. 3A). In this regard, it is of interest that monocytes and MDM ingested similar numbers of opsonized bacteria after 60 min at 37°C (67–75 Ft/100 cells). Comparable data were obtained when Ft were opsonized at 37°C in 10% fresh AS and then washed prior to incubation with phagocytes in serum-free medium (data not shown). The ability of fresh AS to augment phagocytosis was ablated by heating serum to 56°C for 20 min (a treatment known to inactivate complement components). Indeed, data obtained using HI-AS and HI-FBS were indistinguishable.
Fig. 3.
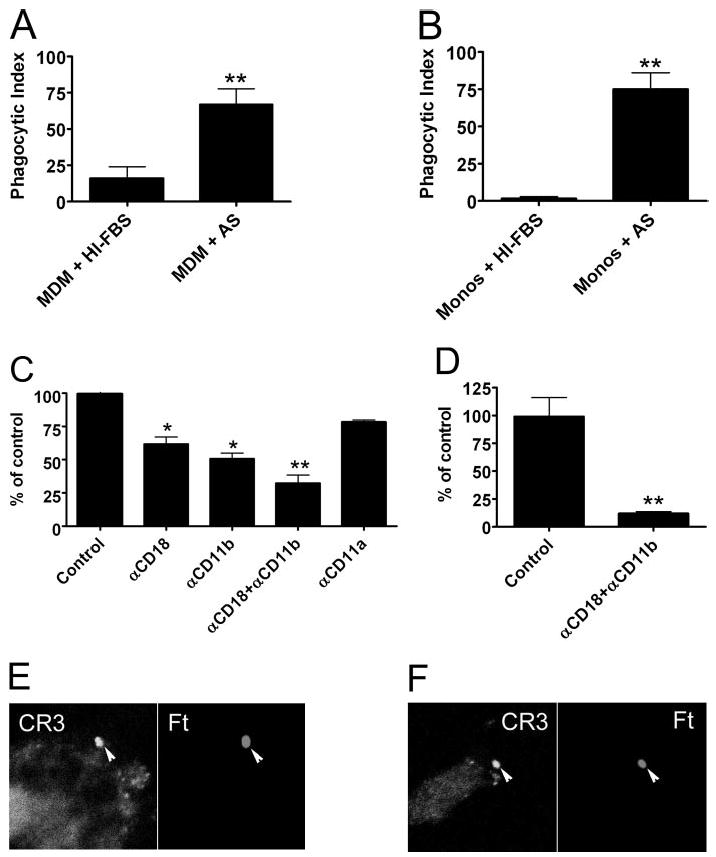
Serum complement is required for optimal phagocytosis of LVS. (A and B) Ft LVS was added at a MOI of 20:1 to MDM (A) or monocytes (Monos; B) in RPMI supplemented with 10% HI-FBS or 2.5% fresh AS as indicated. Phagocytosis was quantified after 1 h at 37°C. Data are the average ± SEM from three to six independent experiments performed in triplicate. **, P < 0.01. (C) MDM in RPMI containing 2.5% AS were left untreated (Control) or incubated with 25 μg/ml anti-CD18, anti-CD11b, anti-CD11a, or a mixture of anti-CD18 and anti-CD11b blocking antibody prior to addition of Ft. Phagocytosis was quantified after 1 h at 37°C. Data are the mean ± SEM from three independent experiments performed in triplicate and are normalized to the no-antibody control (42±7 Ft/100 MDM). *, P < 0.02; **, P < 0.01. (D) Effect of 25 μg/ml anti-CD18 and anti-CD11b antibody on Ft infection of freshly isolated blood monocytes in RPMI containing 2.5% AS. Data are the mean ± SEM from three independent experiments performed in triplicate and are normalized to the no-antibody control (44±4 Ft/100 monocytes). **, P < 0.01. (E and F) Human MDM (E) and monocytes (F) were infected with LVS in RPMI + 2.5% AS for 5 min. Confocal sections show enrichment of CD11b on forming phagosomes (arrowheads).
Confocal analysis demonstrated that CD11b accumulated on forming phagosomes containing opsonized Ft (Fig. 3, E and F), and under these conditions, infection was sensitive to CR3 blockade (Fig. 3, C and D). Anti-CD11b or anti-CD18 blocking antibody reduced phagocytosis of opsonized LVS in a dose-dependent manner (not illustrated), and maximum inhibition was achieved at 25 μg/ml antibody (40–50% inhibition, P<0.02; Fig. 3C). Simultaneous blockade of CD11b and CD18 markedly impaired infection of MDM (~70% inhibition, Fig. 3C) and nearly ablated infection of monocytes (Fig. 3D). Conversely, blockade of lymphocyte function-associated antigen-1 (LFA-1) with mAb to CD11a did not disrupt Ft internalization (P=0.11, Fig. 3C). Taken together, our data document for the first time an important role for serum complement and CD11b/CD18 in Ft infection of human monocytes and confirm the ability of fresh serum to enhance infection of MDM [36].
CR3 does not mediate phagocytosis of unopsonized Ft
CR3 confers uptake of opsonized bacteria by engaging fixed C3bi. However, this receptor can also mediate nonopsonic uptake of certain microbes by binding directly to LPS or other surface factors [37]. Therefore, we examined whether CR3 was involved in phagocytosis of unopsonized Ft. In marked contrast to opsonized bacteria, blockade of CD11b and/or CD18 did not prevent MDM phagocytosis of unopsonized Ft (Fig. 4A, P≥0.53), and CD11b was not detected on forming phagosomes (Fig. 4B). Blocking antibody specific for CD11a were also without effect (Fig. 4A). We conclude that CR3 is dispensable for phagocytosis of unopsonized Ft.
Fig. 4.
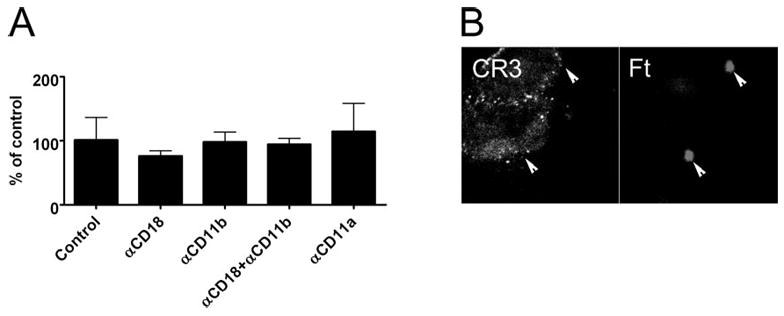
CD11b/CD18 does not mediate phagocytosis of unopsonized Ft. (A) MDM in RPMI containing 10% HI-FBS were left untreated or preincubated with 25 μg/ml anti-CD18, anti-CD11b, anti-CD11a, or a mixture of anti-CD18 and anti-CD11b antibody prior to addition of Ft at a MOI of 20:1, and phagocytosis was quantified after 1 h at 37°C. In all cases, blocking antibodies were without effect (P>0.05). Data are the mean ± SD of triplicate samples from a representative experiment and are normalized to the no-antibody control (6±3 Ft/100 MDM). Similar data were obtained in two other independent experiments. (B) MDM were infected with LVS in RPMI + 10% HI-FBS for 5 min at 37°C. Confocal sections show that CD11b was not recruited to forming phagosomes containing unopsonized bacteria (arrowheads).
Contributions of MR and MBL to phagocytosis of opsonized bacteria
As MRs were enriched on phagosomes containing upopsonized Ft (Fig. 2B) and as CR3 blockade inhibited (but did not prevent) uptake of opsonized bacteria by MDM (Fig. 3C), we assessed the impact of serum opsonins on MR recruitment to sites of Ft engulfment and quantified the role of MR in phagocytosis of opsonized organisms. As shown in Figure 5A, infection of MDM with Ft in medium containing 2.5% fresh AS did not prevent recruitment of MR to forming phagosomes. Nevertheless, under these conditions, ingestion of opsonized Ft by MDM was less sensitive to inhibition by soluble mannan or anti-MR blocking antibody than was phagocytosis of unopsonized bacteria (compare Fig. 5B with Fig. 2A). These data indicate that opsonized Ft engages MR as well as CR3. However, MR was not required for phagocytosis of Ft in media containing active complement components.
Fig. 5.
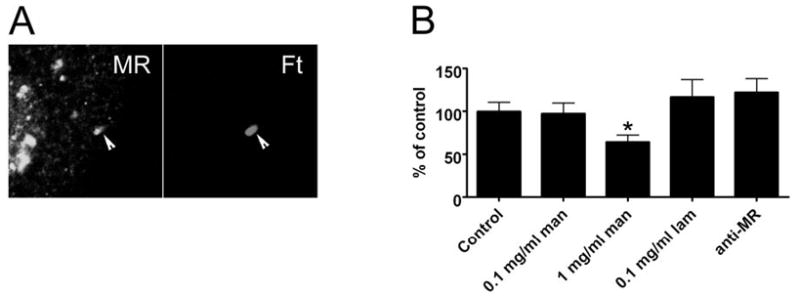
Contribution of MR to uptake of opsonized Ft. (A) MDM were infected with LVS in RPMI + 2.5% AS for 5 min. Confocal sections show MR on forming LVS phagosomes (arrowheads). (B) MDM in RPMI containing 2.5% AS were left untreated (Control) or preincubated with the indicated concentrations of mannan, laminarin, or 10 μg/ml MR blocking antibody prior to addition of Ft at a MOI of 20:1. After 1 h at 37°C, phagocytosis was quantified. Data indicate the mean ± SEM of at least three experiments performed in triplicate for each condition and are normalized to the no-antibody control (102±32 Ft/100 MDM, n=6). *, P = 0.02, versus control.
Whether Ft is opsonized by the lectin pathway or the alternative complement pathway is unknown. The lectin pathway is activated when MBL, an acute-phase reactant, which is sensitive to inhibition by soluble mannan, binds carbohydrates on certain microorganisms [38]. As mannan reduced phagocytosis of opsonized Ft (Fig. 5B), we examined whether these organisms could bind MBL. Using immunoblotting, we could detect MBL on S. aureus, a bacterium known to activate the lectin pathway [39, 40]. By contrast, MBL was not deposited on Ft, which had been incubated with 2.5% or 10% AS at 37°C (data not shown). Our findings suggest that the ability of mannan to impair uptake of opsonized Ft was not a result of competitive inhibition of MBL binding.
DISCUSSION
A central aspect of Ft pathogenesis is the ability of this organism to replicate inside mononuclear phagocytes. Nevertheless, how Ft gains entry into host cells is not well defined. The results of this study indicate a role for the macrophage MR in Ft infection, and as such, our data are the first demonstration of a receptor that binds unopsonized Ft. At the same time, our data provide a molecular explanation for the ability of this microorganism to infect differentially distinct populations of mononuclear phagocytes in the presence and absence of opsonins [5, 6, 12, 16].
Six lines of evidence demonstrate that phagocytosis of un-opsonized Ft is mediated in large part by the macrophage MR. First, MR accumulated on forming Ft phagosomes in MDM. Second, blockade of MR with soluble mannan or specific receptor-blocking antibody significantly impaired Ft binding and internalization, but inhibition of another lectin receptor (dectin-1) did not. Third, the extent of inhibition we obtained for Ft is comparable in magnitude with the effect of mannan on phagocytosis of other pathogens whose uptake is MR-dependent, including L. donovani and M. tuberculosis [21, 41]. Fourth, MR-positive MDM ingested at least fivefold more bacteria than did MR-negative monocytes; and infection was enhanced further by alternative activation, a treatment known to increase MR expression [34]. Fifth, enhanced infection of J774-E cells demonstrated a role for MR in Ft infection of murine macrophages. Finally, differential infection of monocytes, MDM, and J774 cells was not a result of underlying differences in phagocytic capacity, as all three cell types ingested large numbers of H. pylori. Receptors that mediate lectinophagocytosis of H. pylori have not been defined; however, we show here that neither mannan nor laminarin affected uptake of this organism. Similarly, the Ft ligands that engage MR are unknown. One possible candidate is the mannose-rich surface exopolysaccharide capsule [42, 43].
Although the results of this study define an important role for MR in Ft infection, the fact that neither mannan nor anti-MR antibodies ablated Ft entry into MDM suggests that additional, as-yet-unidentified receptors also contribute to Ft internalization. Of interest in this regard are members of the scavenger receptor family [44–46]. These receptors bind a variety of polyanions and mediate internalization of a wide variety of particles including Gram-positive and Gram-negative bacteria [45]. Unlike MR, members of the scavenger receptor family are present on J774 cells and monocytes as well as macrophages [46, 47]. In future studies, it will be important to determine whether scavenger receptors allow monocytes and J774 cells to ingest unopsonized Ft, albeit with low efficiency. Also of interest are lectin receptors of the dendritic cell-specific inter-cellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) family [32]. Like MR, these receptors bind mannose-rich ligands on M. tuberculosis, Leishmania, and other pathogens [32]. Although DC-SIGN is not present on MDM or other resting macrophages [48], murine SIGN-related 1 is present on macrophages in the spleen, and liver/lymph node-specific-SIGN is expressed on a subset of liver and lymph node endo-thelial cells and macrophages [49]. Therefore, it is attractive to predict that a cohort of mannose-binding lectin receptors may play important roles in tularemia via their ability to support infection of a wide variety of cell and tissue types.
Alternative activation of macrophages is distinct from classical activation, which is driven primarily by interferon-γ and enhances microbicidal function [34, 35]. Alternative activation is driven by IL-4 and IL-13 and results in a phenotype of enhanced endocytosis and antigen presentation, which are characterized by increased expression of MR and major histo-compatibility complex Class II as well as production of predominantly anti-inflammatory cytokines [34, 35]. In this regard, it is of interest that alternatively activated macrophages up-regulate DC-SIGN and scavenger receptors in addition to MR, and we show here that IL-4-treated MDM exhibit enhanced phagocytosis of unopsonized Ft. In healthy adults, alternatively activated macrophages are found in the lung [48], and it is generally believed that the distinct phenotype of alveolar macrophages limits potentially damaging inflammation in this locale [50]. The results of this study suggest that alveolar macrophages may be particularly susceptible to Ft infection and if so, could account in part for the low infectious dose of this organism when acquired via the aerosol route. Indeed, published data suggest that murine alveolar macrophages engulf Ft LVS more avidly than other macrophage types and support bacterial replication [51]. In contrast, classically activated MDM retard replication of Ft subsp. novicida by enhancing phagosome-lysosome fusion [7].
Our studies of complement-opsonized Ft are notable for several reasons. First, we show that 2.5% fresh AS was sufficient to confer opsonophagocytosis. Second, CD11b/CD18 accumulated on forming phagosomes and mediated internalization of opsonized Ft, but LFA-1 did not. Third, the extent of inhibition we document here for opsonized Ft is concordant with the effects of CR3-blocking antibodies obtained in other systems [52, 53]. Our data are also in good agreement with the results of a recent study [36], which found that 10% human AB serum enhances MDM infection with opsonized Ft in a CR3-dependent manner. Moreover, we extended the findings of Clemens et al. [36] to show for the first time that freshly isolated human blood monocytes and MDM ingest similar numbers of opsonized Ft and that CD11b accumulated on forming phagosomes only under conditions of opsonization. Moreover, our demonstration that monocyte infection was nearly ablated by CR3 blockade is consistent with the fact that these cells are refractory to infection with unopsonized Ft {(Fig. 1A) and ref. [16]}. Finally, we show that the lectin pathway of complement did not efficiently opsonize Ft.
Like Ft, L. donovani and virulent strains of M. tuberculosis use MR and CR3 to gain entry into macrophages [21, 41, 54]. The ability of several intracellular pathogens to engage these two receptors is significant, as mechanism of entry is an important regulator of microbe fate [17]. Of particular note here is the fact that infection via MR and CR3 is considered a relatively “safe” route of entry, which results in minimal activation of host microbicidal systems. The signaling cascades downstream of these two receptors are not coupled to production of proinflammatory cytokines and at least for CR3, do not trigger a strong respiratory burst [17]. Indeed, MR ligation can actively suppress tumor necrosis factor α (TNF-α) [55], and this may account for the fact that Ft LVS-infected MDM secrete less TNF-α than infected monocytes [16]. Finally, recent data indicate that engagement of MR by M. tuberculosis is required for subsequent inhibition of phagosome-lysosome fusion [33]. Whether MR influences maturation of the Ft phagosome or alters the rate or extent of phagosome escape is unknown. However, the fact that opsonized and unopsonized Ft replicate in human and murine macrophages [5–7], together with the results of this study, support the notion that MR and CR3 are safe portals of entry.
We and others [24, 56] have shown previously that during CR3-mediated phagocytosis, C3bi-opsonized particles sink into the body of the macrophage without elaboration of large pseudopodia. In marked contrast, Clemens et al. [36] found that opsonized Ft are captured by large, asymmetric pseudopodia, which project from the macrophage surface to ensnare bacteria. Taken together, these data suggest that “looping phagocytosis” may not be mediated by signaling pathways downstream of CR3. We show here that opsonized Ft recruits MR and CR3 to forming phagosomes, and as such, our data suggest that signaling downstream of MR may influence phagosome morphology. In support of this notion, periodate oxidation of Ft LPS and capsule prevents looping entry [36], and under these conditions, forming phagosomes exhibit a typical CR3-like morphology, whereas uptake of opsonized Ft, which were killed by other methods, appears unchanged. This issue merits further study, and it is also possible that periodate-sensitive Ft virulence determinants modulate phagosome morphology by receptor-independent mechanisms.
In summary, we have shown that Ft LVS infects MDM more efficiently than peripheral blood monocytes or J774 cells in standard media and demonstrate that infection efficiency is mediated, at least in part, by the ability of unopsonized Ft to engage MR. At the same time, our findings support a model in which MR and CR3 play distinct roles during opsonophagocytosis. Additional studies are needed to define the full array of binding interactions that allow Ft to infect mononuclear phagocytes and to define how differential receptor expression impacts infection and microbe fate in vivo and in vitro.
Acknowledgments
This study was supported by funds from the National Institutes of Health (P01-AI44642) to L-A H. A. We thank Dr. Philip D. Stahl (Washington University, St. Louis, MO) for providing MR-positive J774-E cells, Dr. Michael Apicella (University of Iowa, Iowa City) for LVS, and L. Zhao and G. Chan (University of Iowa Department of Statistics and Actuarial Science) for helpful discussions.
References
- 1.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defense renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 2.Tarnvik A, Berglund L. Tularaemia. Eur Respir J. 2003;21:361–373. doi: 10.1183/09031936.03.00088903. [DOI] [PubMed] [Google Scholar]
- 3.Santic M, Molmeret M, Klose KE, Abu Kwaik Y. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 2006;14:37–44. doi: 10.1016/j.tim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Anthony LD, Burke RD, Nano FE. Growth of Francisella spp. in rodent macrophages. Infect Immun. 1991;59:3291–3296. doi: 10.1128/iai.59.9.3291-3296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004;72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santic M, Molmeret M, Abu Kwaik Y. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-γ. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 8.Lauriano CM, Barker JR, Yoon SS, Nano FE, Arulanandam BP, Hassett DJ, Klose KE. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc Natl Acad Sci USA. 2004;101:4246–4249. doi: 10.1073/pnas.0307690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthony LS, Cowley SC, Mdluli KE, Nano FE. Isolation of a Francisella tularensis mutant that is sensitive to serum and oxidative killing and is avirulent in mice: correlation with the loss of MinD homologue expression. FEMS Microbiol Lett. 1994;124:157–165. doi: 10.1016/0378-1097(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 10.Golovliov I, Ericsson M, Sandstrom G, Tarnvik A, Sjostedt A. Identification of proteins of Francisella tularensis induced during growth in macrophages and cloning of the gene encoding a prominently induced 23-kilodalton protein. Infect Immun. 1997;65:2183–2189. doi: 10.1128/iai.65.6.2183-2189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron GS, Nano FE. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol Microbiol. 1998;29:247–259. doi: 10.1046/j.1365-2958.1998.00926.x. [DOI] [PubMed] [Google Scholar]
- 12.Telepnev M, Golovliov I, Grundstrom T, Tarnvik A, Sjostedt A. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signaling and secretion of TNF-α and IL-1 from murine macrophages. Cell Microbiol. 2003;5:41–51. doi: 10.1046/j.1462-5822.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai XH, Golovliov I, Sjostedt A. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun. 2001;69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai XH, Golovliov I, Sjostedt A. Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb Pathog. 2004;37:225–230. doi: 10.1016/j.micpath.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolger CE, Forestal CA, Italo JK, Benach JL, Furie MB. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J Leukoc Biol. 2005;77:893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- 17.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 18.Allen LA, Schlesinger LS, Kang B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J Exp Med. 2000;191:115–128. doi: 10.1084/jem.191.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung AL, Nast CC, Bayer AS. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect Immun. 1998;66:5988–5993. doi: 10.1128/iai.66.12.5988-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen LAH, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- 21.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 22.Puig-Kroger A, Serrano-Gomez D, Caparros E, Dominguez-Soto A, Relloso M, Colmenares M, Martinez-Munoz L, Longo N, Sanchez-Sanchez N, Rincon M, Rivas L, Sanchez-Mateos P, Fernandez-Ruiz E, Corbi AL. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;279:25680–25688. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 23.Fiani ML, Beitz J, Turvy D, Blum JS, Stahl PD. Regulation of mannose receptor synthesis and turnover in mouse J774 macrophages. J Leukoc Biol. 1998;64:85–91. doi: 10.1002/jlb.64.1.85. [DOI] [PubMed] [Google Scholar]
- 24.Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heesemann J, Laufs R. Double immunofluorescence microscopy technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985;22:168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen LA, Allgood JA. Atypical protein kinase C-ζ is essential for delayed phagocytosis of Helicobacter pylori. Curr Biol. 2002;12:1762–1766. doi: 10.1016/s0960-9822(02)01216-2. [DOI] [PubMed] [Google Scholar]
- 27.Stahl P, Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982;93:49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179–192. doi: 10.1615/critrevimmunol.v24.i3.20. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell JM, Ezekowitz RA, Roberts MB, Channon JY, Sim RB, Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willment JA, Gordon S, Brown GD. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–43823. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 31.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 32.Cambi A, Koopman M, Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 33.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 35.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 36.Clemens DL, Lee BY, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agramonte-Hevia J, Gonzalez-Arenas A, Barrera D, Velasco-Velazquez M. Gram-negative bacteria and phagocytic cell interaction mediated by complement receptor 3. FEMS Immunol Med Microbiol. 2002;34:255–266. doi: 10.1111/j.1574-695X.2002.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 38.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway—its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 39.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 40.Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mono-nuclear phagocytes. Infect Immun. 1988;56:363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherwonogrodzky JW, Knodel MH, Spence MR. Increased encapsulation and virulence of Francisella tularensis live vaccine strain (LVS) by subculturing on synthetic medium. Vaccine. 1994;12:773–775. doi: 10.1016/0264-410x(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 43.Hood AM. Virulence factors of Francisella tularensis. J Hyg. 1977;79:47–60. doi: 10.1017/s0022172400052840. Lond. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gough PJ, Greaves DR, Suzuki H, Hakkinen T, Hiltunen MO, Turunen M, Herttuala SY, Kodama T, Gordon S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- 45.Gough PJ, Gordon S. The role of scavenger receptors in the innate immune system. Microbes Infect. 2000;2:305–311. doi: 10.1016/s1286-4579(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 46.Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defense and disorders of the central nervous system. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jozefowski S, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–8041. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- 48.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 49.Koppel EA, van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 50.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 51.Polsinelli T, Meltzer MS, Fortier AH. Nitric oxide-independent killing of Francisella tularensis by IFN-γ-stimulated murine alveolar macrophages. J Immunol. 1994;153:1238–1245. [PubMed] [Google Scholar]
- 52.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 53.Schlesinger LS, Horwitz MA. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-γ activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–1994. [PubMed] [Google Scholar]
- 54.Zimmerli S, Edwards S, Ernst JD. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–770. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Tachado SD, Patel N, Zhu J, Imrich A, Manfruelli P, Cushion M, Kinane TB, Koziel H. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J Leukoc Biol. 2005;78:665–674. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol. 1977;6:797–807. doi: 10.1111/j.1365-3083.1977.tb02153.x. [DOI] [PubMed] [Google Scholar]


