Abstract
Brain atrophy associated with chronic alcohol consumption is partially reversible after cessation of drinking. Recovering alcoholics (RA, 45±8 years) were studied with MRI within one week of entering treatment, with followup at 8 months. Light drinkers (LD) were studied with MRI twice 1 year apart. For each participant, deformation maps of baseline structure and longitudinal size changes between baseline and followup scans were created using nonlinear registration techniques. ANCOVA assessed group differences and regression methods examined relationships between deformation maps and measures of drinking severity or baseline atrophy. At baseline, RA showed significant atrophy in the frontal and temporal lobes. Longitudinally, abstainers recovered tissue volumes significantly faster than LD in parietal and frontal lobes. When comparing abstainers to relapsers, additional regions with significantly greater recovery in abstainers were temporal lobes, thalamus, brainstem, cerebellum, corpus callosum, anterior cingulate, insula, and subcortical white matter. Gray matter volume at baseline predicted volume recovery during abstinence better than white matter. Drinking severity was not significantly related to brain structural changes assessed with this method. Longitudinally, deformation based morphometry confirmed tissue recovery in RAs who maintain long-term sobriety. Abstinence-associated tissue volume gains are significant in focal parts of the fronto-ponto-cerebellar circuit that is adversely affected by heavy drinking.
Keywords: longitudinal, general linear model, computational anatomy, atrophy, alcoholism, MRI
INTRODUCTION
Neuroimaging methodology, including magnetic resonance imaging (MRI), has been used to study atrophy associated with chronic alcohol dependence (for reviews see e.g., (Rosenbloom et al. 1995; Sullivan 2000; Sullivan et al. 2000). Common findings are enlarged ventricles and sulci (Jernigan et al. 1991; Hayakawa et al. 1992; Pfefferbaum et al. 1992), as well as generalized loss of volume in cortical gray matter (GM), white matter (WM), cerebellum (Shear et al. 1996; Sullivan et al. 2000), and subcortical structures (Jernigan et al. 1991; Jernigan et al. 1991; Pfefferbaum et al. 1992; Charness 1993; Hommer et al. 1995; Sullivan et al. 1995; Pfefferbaum et al. 1996; Sullivan et al. 1996). These studies were usually performed in recovering alcoholics in treatment who, at time of MRI study, were abstinent from alcohol for several weeks to several months. However, baseline atrophy may be underestimated in alcoholics who are abstinent for several weeks, as global brain tissue volume has been reported to recover significantly within just a few weeks of sobriety (Gazdzinski et al. 2005), with the most rapid recovery apparent in those with the greatest baseline atrophy and drinking severity (Pfefferbaum et al. 1995). Thus, in order to describe/capture the full extent of baseline chronic alcohol-induced brain atrophy, it is necessary to study alcoholics as soon as possible after cessation of chronic drinking before significant structural recovery can occur (Gazdzinski et al. 2005), or to study actively drinking individuals with alcohol use disorders (Cardenas et al. 2005).
Nearly all of the previous MRI studies of the effects of alcohol on the brain, including our earlier studies (Di Sclafani et al. 1995; Fein et al. 2002), used automated computer segmentation of relatively low resolution images to subdivide the brain into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF), often combined with manual or automated tracing of regions of interest (ROIs), such as cerebral lobes and specific subcortical brain structures.
Deformation tensor morphometry (Davatzikos et al. 1996; Machado et al. 1998; Gaser et al. 1999; Studholme et al. 2001) is a relatively new MRI processing method that quantifies and visualizes shape differences between brains on a voxel-by-voxel basis without a priori definitions of ROIs. This method should be more sensitive to focal effects of chronic alcohol consumption on brain structure, which is potentially obscured by volumetric quantification of large brain ROIs. As opposed to voxel-based morphometry (VBM, as commonly used in Statistical Parameter Mapping software), which examines image differences that remain after approximate registration, deformation-based morphometry (DBM) aims to detect shape differences directly from consistent patterns in the anatomical displacement fields, deriving measures of shape from registration rather than misregistration. Recent methodological improvements also make registration between serial images obtained from the same individual feasible, leading to direct maps of change over time that can be used for longitudinal DBM. We previously used cross-sectional DBM to describe cross-sectional brain shape changes associated with heavy drinking in treatment-naïve individuals vs. light-drinking controls (Cardenas et al. 2005). The main findings suggest dose-related, focal cortical gray matter losses and diffuse CSF increases in the brains of heavy drinkers, with little-to-no evidence of significant white matter losses.
Chronic alcohol-induced brain tissue loss is at least partially reversible after cessation of drinking, the detection of which may be amenable to serial structural MRI measurements. However, previous studies of brain structure recovery in abstinent alcohol dependent individuals evaluated only changes in relatively large brain regions using quantitative volumetric MRI (Zipursky et al. 1989; Shear et al. 1994; Pfefferbaum et al. 1995; Trabert et al. 1995; Gazdzinski et al. 2005). The voxel-based method of DBM can localize/visualize specific spatial patterns of recovery of focal cerebral dysmorphology in abstinent alcoholics and quantify its extent.
The goals of this study were to describe the application of DBM to brain changes in alcohol-dependent individuals during short- and long-term abstinence from alcohol. We have specifically used DBM which examines the derivatives of the displacement fields in order to extract specific measures of local size rather than location from the mappings between anatomies. Atrophy patterns in 1-week-abstinent alcohol dependent individuals were measured and visualized in comparison to light-drinking controls. Brain morphologic changes during prolonged abstinence (6 – 9 months) and relapse were compared and described as a function of drinking severity and level of atrophy at approximately one week of abstinence. Specifically, we tested the following a priori hypotheses: 1) Alcohol dependence is associated with widespread reductions of parietal and temporal GM volumes, with focal frontal GM loss, and with WM losses primarily in the frontal and parietal lobes; 2) GM and WM volume decreases are related positively to drinking severity; 3) abstinence from alcohol over 6–9 months is associated with significant local brain tissue volume increases primarily in the WM and 4) tissue recovery is greatest in individuals with the greatest alcohol consumption severity and the greatest atrophy at approximately one week of abstinence.
METHODS AND MATERIALS
Participants
Recovering alcoholics (RA) were recruited from the San Francisco VA Medical Center Substance Abuse Day Hospital and the San Francisco Kaiser Permanente Chemical Dependence Recovery Program outpatient clinics, and light drinking controls (LD) responded to advertisements in the community. Prior to completing any procedure, all participants gave written informed consent, which was approved by review boards of the University of California San Francisco and the San Francisco VA Medical Center. All participants had negative breath alcohol levels and urine screens for THC, opiates, PCP, cocaine, or amphetamines at the time of study procedures.
Participants were administered the Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition) by a clinical neuropsychologist, and alcohol consumption history for all participants was obtained using the Lifetime Drinking History (LDH) (Skinner and Sheu 1982; Sobell et al. 1988; Sobell and Sobell 1992). These data yielded several estimates of drinking severity including average drinks/month computed over the past year, average lifetime drinks/mo (computed since beginning regular drinking, i.e., more than 1 drink/mo), and cumulative lifetime alcohol consumption in kg (total number of standard drinks over the lifetime × 0.0136 kg/drink). Substance use history was acquired with an in-house questionnaire assessing substance type, and quantity and frequency of use.
Primary inclusion criteria for RA were current DSM-IV diagnosis of alcohol dependence or alcohol abuse (American Psychiatric Association, 1994), fluency in English, consumption of greater than 150 drinks per month (one drink equivalent = 12 oz of beer, 5 oz of wine, 1.5 oz of liquor, corresponding to approximately 13.6 grams of pure alcohol) for at least 8 years prior to enrolment for men, and consumption of greater than 80 drinks per month for at least 6 years prior to enrolment for women. The LDs currently drank less than 45 drinks per month and averaged less than 45 drinks/mo over their lifetime. Primary exclusion criteria are fully detailed in Durazzo et al. (Durazzo et al. 2004). In brief, all participants were free of general medical (except hepatitis C and hypertension in RA), neurologic, and psychiatric (except unipolar mood or substance use disorders in RA) conditions known or suspected to influence brain morphology. Current or past unipolar mood disorders (e.g., major depression, unipolar substance-induced mood disorders) were not exclusionary for RA given the high comorbidity with alcohol use disorders (Gilman and Abraham 2001).
Cross-sectional study
We studied 47 (44/3 male/female) RA, 45 ± 8 (range 28–67) years of age, within 5.8 ± 2.9 days since their last drink and after detoxification and compared them to 18 (16/2 male/female) LD, 49 ± 14 (range 34–58) years of age. The RA and LD groups did not significantly differ in age, but the LDs had a higher level of formal education than RAs (see Table 1 for demographic information). Seventy-one percent of the RA group were Caucasian and 13 percent were of African American decent. The ethnic representation in the LD group was similar to that of the RA group. The RA group drank on average 403 ± 189 standard drinks/mo over the past year, and the LD group drank 11 ± 10 drinks/mo. Seven RA participants were positive for hepatitis C antibody, 10 had medication-controlled hypertension, and one had mild-moderate chronic obstructive pulmonary disease (emphysema). Three RA participants individually met criteria for past opioid dependence, methamphetamine dependence and cocaine abuse and one for current cannabis abuse. All the RA participants who met criteria for past substance dependence/abuse were in sustained full remission and had not used the substance for at least 5 years prior to enrolment. At the time of enrolment, 9 RA participants were diagnosed with recurrent major depression and 8 RA with substance-induced (alcohol) mood disorder with major depressive or depressive features.
Table 1.
Participant Demographics
| LD N=18 | RA N=47 | |
|---|---|---|
| Age [years] | 45 ± 8 | 49 ± 14 |
| Education* [years] | 17 ± 2 | 14 ± 2 |
| 1 yr Avg drinks/mo* | 11 ± 10 | 403 ± 189 |
| Lifetime Avg drinks/mo* | 17 ± 14 | 240 ± 123 |
| Lifetime kg of Alcohol* | 75 ± 61 | 1251 ± 783 |
RA>LD, p<0.001
Longitudinal study
Twenty-five of 47 RA (age 50 ± 9 years) returned for follow-up study approximately 8 months after their baseline study. Eight LD (age 45 ± 9 years) returned for follow-up study after approximately 12 months. Of the 25 longitudinally studied RA, 17 were abstinent for at least two months before follow-up, for an average of 188±66 consecutive days of abstinence (abstaining RA, AbRA); of these 17 individuals, 12 drank no alcohol at all between studies, whereas five drank at or below their pre-treatment levels for 4 – 126 days. Eight of the 25 RA were defined as relapsers (relapsing RA, ReRA) because they had consumed their most recent alcoholic drinks between 1 and 15 days prior to follow-up MRI ; all of them drank at their pre-treatment levels for 3 – 100 days. Table 2 summarizes the demographics of this longitudinal sample. As shown in Table 2, the abstainers did not differ significantly from the relapsers on any alcohol consumption measure or years of education.
Table 2.
Demographics of participants studied longitudinally
| LD N=8 | AbstainingRA N=17 | RelapsingRA N=8 | |
|---|---|---|---|
| Age at baseline (yrs) | 45 ± 9 (34–58) | 51 ± 11 (28–67) | 48 ± 4 (41–53) |
| Age at followup (yrs) | 46 ± 9 (36–59) | 52 ± 11 (29–67) | 49 ± 4 (42–53) |
| Education (yrs) | 17 ± 3 | 15 ± 2 | 14 ± 3 |
| 1 yr Avg Drinks/Mo | 8 ± 13 | 396 ± 199 | 359 ± 121 |
| Lifetime Avg Drinks/Mo | 17 ± 18 | 211 ± 144 | 246 ± 130 |
| Lifetime kg of Alcohol | 81 ± 79 | 1093 ± 831 | 1198 ± 601 |
| Days since last drink before followup MRI | N/A | 188 ± 66 | 8 ± 6 |
mean ± standard deviation (min-max)
MRI acquisition
MRI data were acquired on a clinical 1.5 Tesla MR scanner (Vision, Siemens Medical Systems, Iselin NJ). The MRI protocol consisted of a double spin-echo (DSE) sequence, yielding proton density and T2 weighted MR images and a Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence, yielding T1 weighted MR images. The axially angulated contiguous DSE images (TR/TE1/TE2 = 2500/20/80 ms, 1 × 1 mm2 resolution, 3 mm slice thickness, no slice gap) were oriented along the orbital-meatal angle + 5° as seen in a sagittal scout image through the brain midsection. The coronal MPRAGE dataset (TR/TI/TE = 9/300/4 ms, 1 × 1 mm2 in-plane resolution, 1.5 mm slabs) was acquired orthogonal to the long axis of the hippocampus.
Deformation Based Morphometry
Creation of maps of atrophy and longitudinal change: An entropy driven B-Spline Free Form deformation algorithm (Studholme et al. 2001; Studholme et al. 2001; Studholme et al. 2003) was used to register individual scans to the reference atlas. For this study, an MRI of a 36-year old male light drinker was used as the reference brain shape for the spatial normalization. The Jacobian determinant of this transformation, giving the fractional volume contraction or expansion at each voxel evaluated, was calculated to give a deformation map at 1 week of abstinence. The Jacobian maps were filtered using an intensity consistent filtering approach (Studholme et al. 2003), where the contribution of each neighboring Jacobian determinant to the center pixel is weighted by a Gaussian spatial kernel with a maximum filter width of 15 mm FWHM and a measure of association based on the observed local co-occurrence of subject and group averaged intensity values after spatial normalization. Deformation maps at 1 week of abstinence were used for cross-sectional comparisons of RA and LD. Robust fluid registration was used to create deformation maps of longitudinal size changes between scans at 1 week of abstinence and follow-up scans of each participant (Studholme et al. 2006). These longitudinal deformation maps were transformed to the common template using the B-Spline deformation for spatial statistical analysis and then smoothed with the same 15 mm filter described above.
Statistical analysis
Statistical measures were applied to locate points where voxel level differences in the Jacobian occurred. ANCOVA was used to test our first hypothesis that alcohol dependence was associated with widespread tissue loss; the deformation maps at 1 week of abstinence were the dependent variable, group status (i.e., RA or LD) was the categorical predictor, and head size was the covariate. Headsize was calculated from the Jacobian for each participant averaged over the manually delineated intracranial region of the reference anatomy (for details see Studholme et al. 2004). Multiple linear regression was used to test whether GM and WM volume decreases were related to drinking severity (hypothesis 2), with the deformation maps at 1 week of abstinence as dependent variables, LDH measures as independent variables, again covarying for head size. We used ANOVA to examine whether there was significant recovery of brain volume during abstinence (hypothesis 3), with the longitudinal deformation maps as dependent variables and group status as the categorical variable. Hypothesis 4, that tissue recovery was related to drinking severity and tissue atrophy at 1 week of abstinence, was tested using multiple linear regression. The longitudinal deformation maps were the dependent variable, LDH measures or global GM and WM volumes at 1 week of abstinence were separately entered as independent variables, controlling for age (to account for GM variability at 1 week of abstinence due to normal aging).
Because statistics were computed independently at each voxel (i.e., the number of statistical comparisons equals the number of voxels), corrections for multiple comparisons were necessary. We calculated the corrected p<0.05 peak threshold using permutation testing (Nichols and Holmes 2002), and also corrected for multiple comparisons by using cluster size tests, where voxels with t-statistics above a predetermined threshold are clustered together if they are neighbors, and clusters exceeding a certain size are deemed significant despite multiple comparisons. Cluster size tests usually assume stationarity or uniform smoothness of the statistical map, and violations of stationarity can result in an increased number of false positives in smooth regions and false negatives in rough regions (Hayasaka et al. 2004). Our DBM data, even after smoothing with our intensity consistent filter, exhibit nonstationarity. Because of this, we used fMRIstat (Worsley et al. 2002) to identify in RA clusters of contracting or expanding voxels using the nonstationary random field test that accounts for local smoothness, where both magnitude (all voxels within the cluster must be significant at P<0.001 uncorrected) and spatial extent (larger clusters are less likely to be false positives) were jointly used to assess corrected significance. The nonstationary random field test has a small computational burden and performs well under high smoothness and high degrees of freedom, conditions which are met in our planned DBM analyses. Statistical maps were displayed using the Rview software (http://rview.colin-studholme.net).
RESULTS
Cross-sectional Findings
RA at 1 week of abstinence vs. LD
RA showed tissue loss compared to LD in the frontal, parietal, temporal, and occipital GM and WM, as well as cerebellum (no figure shown). The threshold at p=0.05 corrected for multiple comparisons using permutation testing is T<−5; no voxels exceed this threshold to show significant atrophy after correction. However, cluster analysis using fMRIstat revealed two clusters where RA participants had consistently smaller tissue volumes than LD individuals. The larger and more significant cluster was located in the dorsolateral prefrontal region and was composed of both GM and WM. The cluster volume was 2112 mm3 (2.1 cc as 1 cc = 10 mm × 10 mm × 10 mm) with corrected p=0.04. The smaller cluster encompassed right temporal GM, had volume of 1277 mm3 (1.3 cc), and corrected p=0.07. To estimate the magnitude of atrophy in RA compared to LD, we averaged the volume group effect estimates from the ANCOVA at all voxels within each cluster: Tissue volume was reduced on average by 19% in the left frontal and by 18% in the right temporal cluster.
RA tissue shape changes vs. alcohol consumption
We separately examined the relationships between tissue volume at 1 week of abstinence in the RA and the number of drinks/mo averaged over 1 year, the lifetime average number of drinks/mo, and the lifetime total kg of alcohol consumed. Negative associations with both lifetime drinking severity measures were observed at the uncorrected p=0.05 threshold, where smaller tissue volumes were linked to greater alcohol consumption, particularly in the frontal and temporal lobes. No single voxel survived peak correction for multiple comparisons, and there were no significant clusters composed of voxels with strongly negative associations (all voxels with p<0.001).
Longitudinal Findings
Tissue shape changes in RA vs. LD
T-statistic maps from 25 RA (AbRA and ReRA) vs. 8 LD thresholded at p=0.05 uncorrected (no figure given) revealed faster tissue volume increase in RA over 6 – 9 months of abstinence in frontal lobe and cerebellar WM and GM, temporal lobe, parietal lobe, and subcortical regions (particularly WM). Occipital lobes showed little if any tissue volume increases. CSF shrinkage was apparent in the interhemispheric fissure, temporal sulci, and ventricles. Most voxels did not survive peak correction for multiple comparisons. However, cluster analysis using fMRIstat revealed two clusters where RA participants, as a group, demonstrated consistently greater tissue increase over 6–9 months than LD. The larger cluster was located in the parietal lobe near the midline, had volume of 1248 mm3 (1.3 cc), corrected p=0.02, and tissue expanding 7% faster in RA compared to LD. The other cluster showed volume increase in the orbital frontal cortex, had volume of 940 mm3 (.9 cc), corrected p=0.02, and tissue expanding 12% faster in RA.
Tissue shape changes as a function of duration of abstinence in RA
The RA group contained participants who drank very proximal to the follow-up MRI, which may partially explain why RA as a group failed to demonstrate greater longitudinal tissue increases relative to LD. We suspected that alcohol consumption within one month of the follow-up MRI in RA would likely arrest or hinder brain volume recovery (Gazdzinski et al. 2005). To test this, we used DBM to compare the 17 AbRA participants who had abstained from alcohol for two months or more before follow-up (average 188 ± 66 days, median 193 days) to 8 ReRA who had consumed their last alcoholic drinks between 1 and 15 days prior to follow-up MRI. Figure 1 shows the t-statistic map from this comparison overlaid on the corresponding average spatially normalized MR images. The red-yellow colors show regions where AbRA recovered tissue volumes at significantly faster than ReRA (see upper wedge of color bar). The threshold at p=0.05 corrected for multiple comparisons using permutation testing is T>5.63, which was exceeded by voxels in the superior anterior frontal lobe. Cluster analysis using fMRIstat revealed ten clusters where AbRA were recovering tissue significantly faster than ReRA, with all p<0.005. The contours of these clusters are overlaid in different colors on the average and t-statistic images in Figure 1. Tissue recovery is found throughout many different brain regions, including the right and left temporal GM and WM, hippocampus, bilateral thalamus, brainstem, cerebellum, anterior corpus callosum and adjacent anterior cingulate GM, anterior and middle frontal GM and WM, insula, temporo-occipital GM, and subcortical WM. In these regions, tissue recovery was 7–12% faster in the AbRA than ReRA, with the greatest difference observed in the 102 cc large region encompassing the medial temporal lobe, thalamus, brainstem, and cerebellum. Table 3 lists the volumes, associated p-values, effect magnitude, and contour colors (shown in Figure 1) for these clusters of tissue volume increase. Figure 2 is a scatterplot of the average tissue recovery, computed using all voxels within the clusters listed in Table 3, for the light drinkers, relapsers, and abstainers. This scatterplot shows that on average, the relapsers were losing tissue volume between scans (−3.1±2.8%), the light drinkers were stable (0.1±1.3%), and the abstainers were gaining tissue (7.7±6.7%). This same pattern was present in all clusters (% tissue change in ReRA<LD<AbRA). Increases in tissue volume over time were also observed in the caudate and lenticular nuclei, but these regions did not survive correction for multiple comparisons. Greater shrinkage (shown in blue) were apparent in the CSF of the interhemispheric fissure, ventricles, many sulcal regions, and in CSF spaces surrounding the cerebellum and brainstem.
Figure 1.
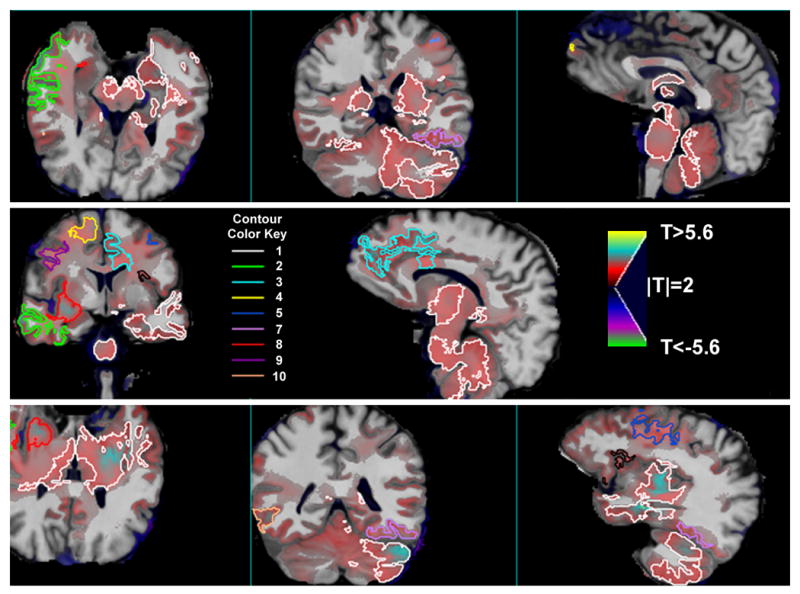
Maps of longitudinal tissue recovery in which 17 abstaining alcoholics are compared to 8 relapsing alcoholics; the T-statistic maps from this comparison are shown here overlaid on the spatially normalized average anatomy (N=33). Red/yellow indicates voxels where abstainers recovered tissue faster than relapsers. The contours encompass clusters of voxels that significantly differentiate abstainers from relapsers after correction for multiple comparisons; see Table III for the cluster numbers corresponding to the contour color key. The contour for cluster 6 is black.
Table 3.
Regions where abstaining RA recovered tissue faster than relapsing RA
| Cluster Number | Cluster Volume (mm3) | p-value | Effect Magnitude (% A >R) | Location | Contour Color |
|---|---|---|---|---|---|
| 1 | 101649.0 | <0.0001 | 11.8 | Left medial temporal lobe, bilateral thalamus, brainstem, cerebellum | White |
| 2 | 13056.0 | <0.0001 | 9.3 | Right anterior temporal lobe | Green |
| 3 | 7554.0 | <0.0001 | 8.3 | Left anterior cingulate | Light blue |
| 4 | 10188.0 | <0.0001 | 8.6 | Right superior anterior frontal lobe | Yellow |
| 5 | 4864.0 | 0.0003 | 8.4 | Left lateral frontal lobe | Blue |
| 6 | 2815.0 | 0.0003 | 9.8 | Left insula | Black |
| 7 | 5553.5 | 0.0004 | 9.9 | Left temporo-occipital lobe | Lavender |
| 8 | 4101.0 | 0.0004 | 7.2 | Deep right frontal white matter | Red |
| 9 | 4084.5 | 0.001 | 8.1 | Right lateral frontal lobe | Purple |
| 10 | 2428.5 | 0.003 | 8.0 | Right temporo-occipital lobe | Peach |
Figure 2.
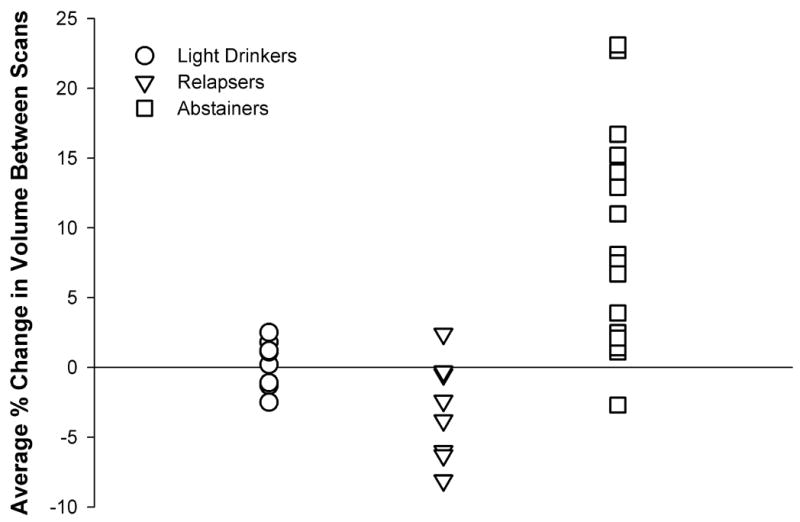
Scatterplot of the average change in % tissue volume between scans, computed using all voxels within the ten significant clusters listed in Table 3.
Longitudinal tissue change in RA vs. alcohol consumption
We regressed longitudinal voxel-wise volume changes in our 25 RA onto measures of drinking severity. We observed positive relationships with drinks/month over one year before abstinence in the left frontal cortex, with lifetime drinks/month in the right anterior cingulate, and with lifetime kg of alcohol in the frontal GM bilaterally. No single voxel survived peak correction for multiple comparisons, and there were no significant clusters composed of voxels with strongly positive associations (all voxels with p<0.001).
Longitudinal tissue change vs. GM or WM volume at 1 week of abstinence
Figure 2 shows regions of the brain where tissue volume recovery was related to tissue volume at 1 week of abstinence, with t-statistics overlaid on the spatially normalized MRI. RA with less global GM at 1 week of abstinence had significantly greater GM recovery in frontal lobe, most prominently in the anterior cingulate and adjacent frontal WM and GM (Figure 3). The threshold at p=0.05 corrected for multiple comparisons using permutation testing is |T|>6; no peak voxels were significantly related to GM at 1 week of abstinence after correction. Six significant clusters above p=0.001 were identified primarily in the frontal lobe, and specifically in the anterior and middle cingulate gyrus, inferior, superior, and lateral frontal GM; one relatively small cluster was also found in the right anterior temporal lobe. In all regions, the tissue recovery was between 10 and 21% faster for each 10% reduction in global %GM at baseline. Table 4 shows the volumes, associated p-values, effect magnitude, and contour color (used in Figure 2) for these clusters. In contrast to global GM at baseline, tissue recovery was not strongly related to global WM at 1 week of abstinence, as no significant peak differences or clusters were observed above p=0.001.
Figure 3.
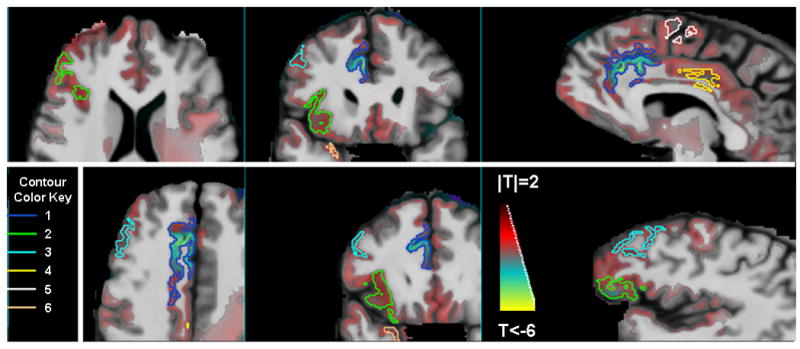
The T-statistic map, showing the relationship between longitudinal tissue change in 25 recovering alcoholics and gray matter volume at 1 week of abstinence, is shown overlaid on the spatially normalized average anatomy. Red/yellow indicates voxels where smaller GM volumes at 1 week of abstinence were associated with faster tissue recovery. The contours encompass clusters of voxels with this negative association that are significant after correction for multiple comparisons; see Table IV for the cluster numbers corresponding to the contour color key.
Table 4.
Regions where tissue recovery is related to GM at 1 week of abstinence
| Cluster Number | Cluster Volume (mm3) | p-value | Effect Magnitude (%increase in longitudinal change per 10% decrease in GM at 1 week of abstinence) | Location | Contour Color |
|---|---|---|---|---|---|
| 1 | 4486.5 | <0.0001 | 18.1 | Right anterior cingulate | Blue |
| 2 | 3531.0 | 0.0032 | 20.5 | Right inferior frontal | Green |
| 3 | 1092.0 | 0.004 | 12.8 | Right lateral frontal GM | Light blue |
| 4 | 453.0 | 0.01 | 9.7 | Middle cingulate gyrus | Yellow |
| 5 | 460.5 | 0.04 | 14.8 | Midline superior frontal lobe | Light green |
| 6 | 519.0 | 0.05 | 11.6 | Right anterior temporal lobe | Peach |
DISCUSSION
We used deformation-based morphometry to examine brain structure differences between 1-week-abstinent alcoholics and light drinkers, and to compare tissue recovery over several months in abstinent alcoholics to normal aging. The major findings of this study are: 1) alcohol dependence in this predominantly male Caucasian cohort is associated with significant reductions of tissue in foci within the frontal and temporal lobes, 2) heavier drinking is associated with greater tissue losses in the frontal and temporal lobes, although these relationships were not significant after extensive correction for computing statistics at every voxel, 3) RA with at least two months of abstinence demonstrated greater tissue gain throughout the brain than LD or RA who consumed alcohol within 15 days of their follow-up MRI, and 4) tissue recovery for RA as a group was significantly related to GM volume at 1 week of abstinence, but not to WM volume at 1 week of abstinence or to previous drinking severity.
DBM is perfectly suited to visualize brain shape differences between groups, changes over time related to the effects of alcoholism or abstinence on the brain, and to highlight patterns of altered brain structure related to these conditions. The cross-sectional findings expand on previous reports of cerebral abnormalities in chronic alcohol exposure, in that they localize the specific pattern of cerebral dysmorphology. We observed tissue loss in RA in all lobes of the brain, as reported previously using conventional lobar volume measures in treatment-naïve heavy drinkers and in treatment-seeking alcoholics. However, using DBM, only foci in the frontal and temporal lobes reached significance after correction for multiple comparisons. These findings could arise if alcohol leads to spatially diffuse tissue loss that varies across participants, since DBM is best suited for discerning focal losses of brain tissue that occur consistently from one participant to the other in similar brain regions (i.e., spatially consistent). Alternatively, the magnitude of losses in non-significant brain regions may be small, and we do not have the power to detect significant changes.
In this RA cohort, volumes of caudate, putamen, or nucleus accumbens were not significantly reduced as has been previously reported in alcoholics (Sullivan et al. 2005). This may in part be due to imperfect registration of these structures. The sensitivity of crosssectional DBM to detect changes is reduced in regions of high anatomical variability which prevent the estimation of a true one to one mapping between MRI scans of a subject (for example where there are different numbers of sulci and gyri), and may be visualized as blurrier regions in the average spatially normalized image (see Figures 1 and 3). We have implemented automated atlas-based voluming of the caudate, lenticular nuclei, and thalami based on the between-subject registration methods used in this study. In previous work, we showed this automated method had comparable reliability to between-rater manual delineation (Cardenas et al. 2005), but performed least accurately identifying the caudate. If the caudate nuclei were imperfectly registered in our RA, then any tissue contraction reflected in the Jacobian would not consistently map to the same location within the common coordinate system, and our ability to detect volume reductions in RA would be compromised. Alternatively, the filter width used to smooth our Jacobian maps (15 mm) may not have been optimal for detecting atrophy in very small structures such as the nucleus accumbens, reducing the sensitivity of DBM to detect change. Although our previous work has shown that this filter width is optimal for valid estimation of left temporal lobe gyral tissue volumes (Studholme et al. 2004), it may not be optimal for other regions of the brain. In general, the optimal filter width is related to the spatial scale of the effect. For example, large filter widths optimally detect diffuse effects throughout a major lobe, and may blur very focal effects. Investigation of a range of filter widths may reveal other regions of the brain with alcohol-related atrophy.
Within the RA, frontal and temporal lobe volumes were inversely related to measures of alcohol consumption, though not statistically significant after correction for multiple comparisons. This general pattern suggests that greater consumption of alcohol is associated with greater dysmorphology. In a previous DBM study of active heavy drinkers with alcohol use disorders (Cardenas et al. 2005), we only observed association between frontal GM and drinking severity. The RA participants in this study drank nearly twice as heavily in the year prior to study as the active heavy drinkers in our previous study, and it is possible that the temporal region is only affected under this significant level of alcohol consumption. In other words, our results suggest that the frontal lobe is most vulnerable to the effects of heavy chronic alcohol consumption, whereas other brain regions are only affected with more severe drinking levels.
We measured the change in brain volume over time directly by registering images acquired at two time points and evaluating properties of the transformation. Because of the similarity between scans of the same subject, the registration process is simplified, resulting in increased sensitivity and accuracy of longitudinal DBM to capturing change within a subject. When compared to normal aging (i.e., longitudinal tissue change in LDs), RA, as a group, showed greater tissue volume increase over time in many regions throughout the brain, including the frontal lobe and cerebellum, parts of the fronto-ponto-cerebellar circuitry that were hypothesized to be affected by heavy drinking (Sullivan 2003), and are implicated in executive functions, learning and memory, in addition to postural stability and gross and fine motor functions (Sullivan et al. 2005). Most of these regions did not survive correction for multiple comparisons, however, so we compared RA who maintained sobriety to those who relapsed, assuming that relapse would impede tissue recovery. The regions of significantly greater tissue recovery encompassed by contour lines in Figure 1 generally include the same lobar regions shown to recover in our preliminary volumetric ROI analyses of a similar patient cohort (Gazdzinski et al. 2004), and would be consistent with earlier studies that report improvement in global measures of brain volume during abstinence. Figure 1 also displays relatively large colored regions of the images that are not circumscribed by contour lines prescribing significant clusters (e.g., subcortical structures, deep white matter, particular lobules of vermian regions), where the magnitude of tissue gain may be small and we lack the power to detect significant changes. Longitudinal DBM can probe for small-scale changes in local brain volume and separate them from local effects of tissue displacement that can confound techniques such as the boundary shift integral (Boyes et al. 2006). Because of this advantage, DBM adds significant value over conventional methods that previously looked for longitudinal changes in the brain globally or in specific regions, which may not conform to the actual pattern of effect present in the brain. There is also great value in the new hypotheses that are generated based on the results from DBM analyses, such as greater tissue recovery in subcortical structures, that can be used to guide new research.
The findings that brain tissue volume recovers during abstinence and is impeded by relapse are somewhat weakened by our sample size and our definitions of ReRA and AbRA. Our 17 AbRA had maintained uninterrupted sobriety for at least two months before followup; however, 5 of them had a brief episode of drinking between scans. Our ReRA group consisted of recent relpasers, who had consumed alcohol between 1 and 15 days before followup MRI, with the amount of alcohol consumed and the duration of the relapse episode rather variable. The influence of these factors on tissue recovery is unknown and can only be addressed in significantly larger homogeneously relapsing populations.
Within the RA, we examined whether measures of drinking severity and tissue volume at 1 week of abstinence were related to subsequent tissue recovery during abstinence. Our reasoning was based on previous observations by others and us (Pfefferbaum et al. 1995; Gazdzinski et al. 2005) that those with the greatest deficits at 1 week of abstinence (due to heaviest drinking or as indexed by tissue atrophy) might show the greatest recovery. Heavier drinking before alcoholism treatment was not strongly related to subsequent tissue recovery. Tissue recovery per se and its correlation with greater drinking severity may be limited to a relatively young alcoholic population with enough residual neuroplasticity (e.g., local increases of dendritic branching and/or myelin density) to allow appreciable tissue volume increase with prolonged abstinence from alcohol. We found significant relationships between volume of GM at 1 week of abstinence and tissue recovery in the frontal, temporal, and cingulate GM. WM volume at 1 week of abstinence was not related to tissue recovery in any region. This suggests that GM atrophy at entry into alcoholism treatment predicts volume recovery during abstinence better than WM volume at entry. Regression toward the mean is another possible but unlikely interpretation for this finding, since we have previously shown that the errors in DBM-derived volume measurements (compared to manual tracing) are small in comparison to normal subject measurement variability (Studholme et al. 2004).
In this DBM study, the baseline deformation and longitudinal deformation maps were derived from different registration methods. Spatial normalization between subjects, and registration to track change over time within a subject, are two very different registration tasks. We used a fluid registration within subject over time in order to capture subtle sub-voxel changes in tissue boundaries. Although fluid registration can also capture large scale changes, it is prone to problems of stability, allowing large scale flows to occur when estimating mappings between different subjects. We have therefore used B-Spline based deformations in this study for the process of spatial normalization and cross-sectional morphometry. We have applied identical spatial filtering for both analyses to remove ambiguities in the location of size differences or changes due to spatial normalization. Because the spatial normalization process and analysis was identical for all subjects, there should be no significant effects on the mapping and no bias in the group comparisons due to using different registration methods.
In conclusion, DBM is useful for visualization and quantitation of brain shape differences between alcohol-dependent samples and light drinking controls as well as for demonstration of structural alterations during abstinence from chronic alcohol consumption. The results expand on previous observations and complement previous volumetric evaluations of ROIs by these and other investigators. Future work will expand the use of DBM to alcoholic subpopulations (e.g., smokers), investigation of tissue recovery as it relates to regional GM and WM volumes, and examination of brain anatomy underlying neurocognitive deficits observed in recovering alcoholics.
Acknowledgments
This work was supported by R01 AA10788 (D.J.M.), R01 MH65392 (C.S.) and Whitaker foundation award RG-01–0115 (C.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boyes RG, Rueckert D, Aljabar P, Whitwell J, Schott JM, Hill DL, Fox NC. Cerebral atrophy measurements using Jacobian integration: comparison with the boundary shift integral. Neuroimage. 2006;32(1):159–169. doi: 10.1016/j.neuroimage.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Chao LL, Blumenfeld R, Song E, Meyerhoff DJ, Weiner MW, Studholme C. Using automated morphometry to detect associations between ERP latency and structural brain MRI in normal adults. Hum Brain Mapp. 2005;25(3):317–327. doi: 10.1002/hbm.20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138(2):115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Charness ME. Brain Lesions in Alcoholics. Alcoholism: Clinical and Experimental Research. 1993;17(1):2–11. doi: 10.1111/j.1530-0277.1993.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Vaillant M, Resnick SM, Prince JL, Letovsky S, Bryan RN. A computerized approach for morphological analysis of the corpus callosum. J Comput Assist Tomogr. 1996;20(1):88–97. doi: 10.1097/00004728-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Ezekiel F, Meyerhoff DJ, MacKay S, Dillon WP, Weiner MW, Fein G. Brain atrophy and cognitive function in older abstinent alcoholic men. Alcohol Clin Exp Res. 1995;19(5):1121–1126. doi: 10.1111/j.1530-0277.1995.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28(12):1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment--naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26(4):558–564. [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research. Neuroimage. 1999;10(2):107–113. doi: 10.1006/nimg.1999.0458. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Volumes of anatomically defined brain structures on MR images increase with continued abstinence from alcohol; ISMRM Twlfth Scientific Meeting; Kyoto, Japan. 2004. [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78(3):263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29(8):1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63(3):277–286. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Kumagai H, Suzuki Y, Furusawa N, Haga T, Hoshi T, Fujiwara Y, Yamaguchi K. MR imaging of chronic alcoholism. Acta Radiol. 1992;33:201–206. [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22(2):676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Ragan P, Williams W, Rio D, Eckardt M. Decreased cross-sectional area of the corpus callosum in young female alcoholics: An MRI study. Alcoholism: Clinical and Experimental Research. 1995;19:95A. [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15(3):418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Schafer K, Butters N, Cermak LS. Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology. 1991;4(3):175–183. [PubMed] [Google Scholar]
- Machado AM, Gee JC, Campos MF. Atlas warping for brain morphometry. In proceedings of Medical Imaging. SPIE Press. 1998:642–651. [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond J, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: An MRI study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16(6):1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Structural Brain Alterations Associated with Alcoholism. Alcohol Health & Research World. 1995;19(4):266–272. [PMC free article] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics [published erratum appears in Alcohol Clin Exp Res 1994 Jun;18(3):766] Alcohol Clin Exp Res. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res. 1996;20(8):1489–1495. doi: 10.1111/j.1530-0277.1996.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43(11):1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past [published erratum appears in J Stud Alcohol 1989 Jan;50(1):92] J Stud Alcohol. 1988;49(3):225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Blumenfeld R, Schuff N, Rosen HJ, Miller B, Weiner M. Deformation tensor morphometry of semantic dementia with quantitative validation. Neuroimage. 2004;21(4):1387–1398. doi: 10.1016/j.neuroimage.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Maudsley A, Weiner M. An intensity consistent filtering approach to the analysis of deformation tensor derived maps of brain shape. Neuroimage. 2003;19(4):1638–1649. doi: 10.1016/s1053-8119(03)00183-6. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Schuff N, Rosen H, Miller B, Weiner M. Detecting Spatially Consistent Structural Differences in Alzheimer's and Fronto Temporal Dementia Using Deformation Morphometry; Proceedings of Medical Image Computing and Computer Assisted Interventions; Utrecht. 2001. [Google Scholar]
- Studholme C, Cardenas V, Weiner M. Multi-Scale Image and Multi-Scale Deformation of Brain Anatomy for Building Average Brain Atlases. SPIE Medical Imaging Conference 2001 [Google Scholar]
- Studholme C, Drapaca C, Iordanova B, Cardenas V. Deformation based mapping of volume change from serial brain MRI in the presence of local tissue contrast change. IEEE Transactions on Medical Imaging. 2006;25(5):626–639. doi: 10.1109/TMI.2006.872745. [DOI] [PubMed] [Google Scholar]
- Sullivan E. Compromised Pontocerebellar and Cerebellothalamocortical Systems: Speculations on Their Contributions to Cognitive and Motor Impairment in Nonamnesic Alcoholism. Alcohol Clin Exp Res. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. NIAAA Research Monograph No. 34: Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57(7):768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14(3):341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging, chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(1):110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcoholism: Clinical and Experimental Research. 1996;20:348–354. doi: 10.1111/j.1530-0277.1996.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Brain Vulnerability to Alcoholism: Evidence from Neuroimaging studies. NIAAA 2000 [Google Scholar]
- Trabert W, Betz T, Niewald M, Huber G. Significant reversibility of alcoholic brain shrinkage within 3 weeks of abstinence. Acta Psychiatr Scand. 1995;92(2):87–90. doi: 10.1111/j.1600-0447.1995.tb09548.x. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KC, Pfefferbaum A. MRI study of brain changes with short-term abstinence from alcohol. Alcohol Clin Exp Res. 1989;13:664–666. doi: 10.1111/j.1530-0277.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]


