Abstract
The existence and location of undifferentiated cells with the capability of maintaining the homeostasis of the adrenal cortex have long been sought. These cells are thought to remain mostly quiescent with a potential to commit to self-renewal processes or terminal differentiation to homeostatically repopulate the organ. In addition, in response to physiologic stress, the undifferentiated cells undergo rapid proliferation to accommodate organismic need. Sufficient adrenocortical proliferative capacity lasting the lifespan of the host has been demonstrated through cell transplantation and enucleation experiments. Labeling experiments with tritium, BrdU, or trypan blue, as well as transgenic assays support the clonogenic identity and location of these undefined cells within the gland periphery. We define undifferentiated adrenocortical cells as cells devoid of steroidogenic gene expression, and differentiated cells as cells with steroidogenic capacity. In this review, we discuss historic developmental studies together with recent molecular examinations that aim to characterize such populations of cells.
Keywords: stem cell; progenitor cell; NR5A1 (SF1, AD4BP); NR0B1 (DAX1, AHCH); inhibin; differentiation
Adrenal Anatomy and Developmental Milestones
The development of the adrenal gland occurs in discrete phases. During the 4th week of human gestation, coelomic epithelial cells and/or the underlying mesonephric mesenchymal cells residing between the primitive urogenital ridge and the dorsal mesentery begin to proliferate and migrate from the mesonephros to form the earliest manifestation of developing steroidogenic tissue, the adrenogonadal primordium (Sucheston and Cannon 1968; Mesiano and Jaffe 1997). The caudal aspect of this structure, the adrenal primordia is organized into cord-like ultra-structures consistent with steroidogenic potential. This developmental program culminates with the separation of the gonadal and adrenal primordia into discrete fetal organs by the 8th week of gestation (Sucheston and Cannon 1968; Mesiano and Jaffe 1997).
Following the formation of the early adrenal primordia, referred to as the fetal adrenal zone (fetal cortex), the development of the definitive zone (definitive cortex) is initiated. While early researchers assumed that the definitive cortex arose from the fetal cortex, histological observations indicated that the adult/definitive cortex arises from the migration of a distinct population of cells from the coelomic epithelium and/or the underlying mesonephric mesenchyme (Keene and Hewer 1927; Uotila 1940). Moreover, the mesenchymal capsule begins to coalesce around the main portion of the fetal cortex as a loose condensation of flattened cells over the surface of the gland, only later followed by the development of the definitive cortex between the 8th and 9th weeks of gestation (Uotila 1940; Mesiano and Jaffe 1997). The encapsulated gland maintains relatively constant morphology until 16–20 weeks of gestation, at which point growth of fetal zone cells with steroidogenic morphology takes place (Mesiano and Jaffe 1997). The definitive cortex does not undergo differentiation until the 30th week of gestation when this zone eventually differentiates into the zF and zG. Following birth, significant remodeling of the adrenal cortex occurs via regression of the fetal cortex through apoptosis and concomitant expansion of the pre-existing zG and zF (Mesiano and Jaffe 1997). This process of definitive zone expansion occurs by centripetal turnover of outer definitive cortical cells undergoing mitosis. See Figure 1.
Figure 1.
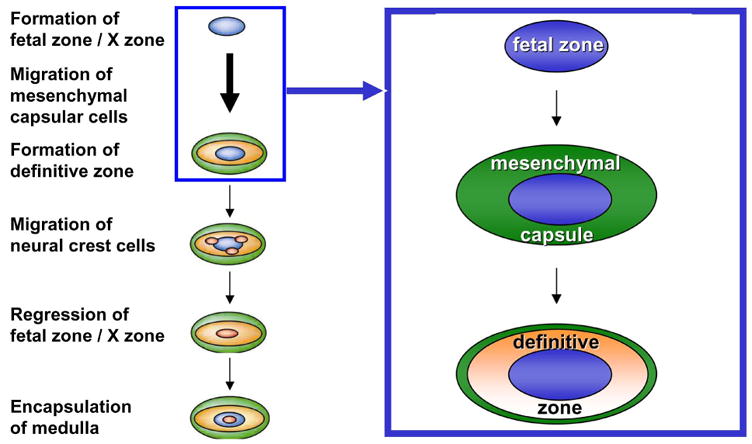
Model of mammalian adrenal organogenesis detailing the sequential formation of the fetal zone, mesenchymal capsule and definitive zone followed by migration and differentiation of neural crest cells into chromaffin cells of the adrenal medulla and postnatal regression of the fetal zone.
The development of the mouse adrenal gland follows similar developmental arrangements. During the gestation period of the mouse, the adrenogonadal primordia arise from the urogenital ridge coelomic epithelium / underlying mesonephric mesenchyme by e9.0, initially marked by the expression of Steroidogenic factor-1 (Sf1) (Luo et al. 1994; Else and Hammer 2005). A discrete adrenal primordium becomes clearly detectable by e12.0 as a collection of cells dorsal and medial to the mesonephros and gonads. The adrenal gland becomes encapsulated followed by continued growth due to the development of a definitive zone. By e16.0–16.5, the adrenal gland contains distinct cortical and medullary compartments. Although the developing mouse fetal adrenal gland contains two distinct cortical zones, it was previously described as lacking a true fetal zone (Else and Hammer 2005). A recent study examining this phenomenon led to the identification of the fetal adrenal enhancer element located in the 4th intron of the Sf1 gene (Zubair et al. 2006). Lineage tracing using a reporter under the control of the fetal enhancer revealed that the fetal cortex in the mouse is present in the mouse embryo as the caudal aspect of the adrenogonadal primordia and is maintained after birth as the X-zone (Zubair et al. 2006). The development of this fetal zone/X-zone only becomes histologically evident at 10–14 days postnatally due to initial intermingling of fetal cortical and medullary structures. Subsequently in males, the zone undergoes regression during sexual maturity and is absent at 38 days of age. In females, fetal zone/X-zone regression takes place during the first pregnancy. Refer to Figure 1.
Adrenocortical Regeneration
The persistent proliferative capacity of the definitive cortex and the observation of centripetal cellular turnover within the cortex support a centripetal-repopulation model of adrenocortical cytogenesis and suggests the possible existence of stem-like cells in the outer compartment of the gland (Mesiano and Jaffe 1997). Following adrenal enucleation, (removal of the inner content of the adrenal gland leaving the only capsule and underlying subcapsular cells intact), the cortex undergoes a dynamic process regeneration (Skelton 1959; Perrone et al. 1986). By the 8th day following enucleation, foci of newly formed cells spread out beneath the capsule and extend towards the center of the gland. Cells continually proliferate from the capsular/subcapsular region to repopulate the newly forming cortex. The regeneration takes approximately 30 days until the gland assumes a normal histological appearance with proper cortical zonation. This regenerated gland recapitulates the normal steroidogenic functions of the adrenal cortex. Transplantation of primary adrenocortical cells results in the formation of similarly functional adrenocortical tissue within the host animal (Thomas et al. 1997; Thomas et al. 2000). The host animal, often adrenalectomized, survive with physiologic replacement of adrenal function by the transplanted tissue (Thomas et al. 1997; Thomas et al. 2000). Moreover, the tissue resulting from these transplantation studies assumes normal adrenocortical architecture (Thomas et al. 1997; Thomas et al. 2000). Hence, both enucleation and transplantation models support a model whereby a pool of cells within the adrenal cortex have the ability to regenerate and replenish the adrenal cortex continually throughout the life of the organism. From these studies, it can be presumed that the outer cortical cells are responsible for this replenishment.
Origin of the Repopulating Cells
The discrete origin of the peripheral cells that repopulate the regenerated gland remains controversial with three working hypotheses. The first, based on the existence of functionally distinct zones, posits that each zone of the adult adrenal gland maintains its own population of cells through proliferation of cells within the individual zone. However, the predominant occurrence of mitosis and proliferation in the subcapsular region and majority of apoptosis occurring in the reticularis/medullary boundary provides qualitative evidence against this theory (Zajicek et al. 1986). Moreover, enucleation experiments suggest that it is not possible for each zone to maintain its own population of cells.
The second hypothesis contends that repopulating cells arise from the mesenchymal capsule. The concept of fibroblast-like capsular cells, serving as the pool of undifferentiated cells that repopulate the gland, is predicated on a series of cytological studies by Zwemer and colleagues, who conducted a lineage tracing study using trypan blue pulse-chase paradigm to track the turnover of blue stained adrenocortical cells over time (Zwemer et al. 1938). After an early exclusive labeling of capsular cells, blue labeled cells were later found in the zG with clearing of capsular staining at later timepoints (Salmon and Zwemer 1941). In addition, early histological studies place adrenocortical cell origin in proximity to cellular columns/projections arising from the capsule (Baker 1952). Moreover, histological evidence indicates differentiation of SF-1 positive steroidogenic cells within areas of SF-1 negative spindle cell hyperplasia of the capsule that extends into the outer parenchyma of zG (Bielinska et al. 2003). Lastly, coincident with ACTH-induced proliferation of the adrenal cortex, proliferating cells marked by PCNA immunohistochemical staining are observed in the capsule eventually repopulating centripetally into the gland (Beuschlein et al. 2002; Pignatelli et al. 2002).
The third hypothesis, which is not incompatible with the second, maintains that undifferentiated subcapsular cells give rise to the rest of the adrenal cortex. In early experiments, mitosis was seen most predominantly in the subcapsular region (Race and Green 1955). Later, pulse chase experiments involving radioactive-thymidine or BrdU studies reveal a predominant labeling of subcapsular cells followed by labeling in cells that are increasingly centripetal and exclusively in the inner cortical zones (Wright et al. 1973; Zajicek et al. 1986). More recent molecular tracking studies support the process of centripetal and clonogenic repopulation of the adrenal cortex (Ford and Young 1963; Iannaccone et al. 2003). Chimeric and transgenic mice harboring a β-galactosidase reporter, under the control of several adrenocortical-specific promoters, reveal radial expression of the transgene from the outer zG to inner zF (Weinberg et al. 1985; Iannaccone et al. 2003). Taken together, these studies suggest that the adrenal parenchyma is composed of clonally expanded cells that extend centripetally in a cord-like fashion from the outermost layers of the cortex to the innermost cortico-medullary boundary. These data support a model whereby peripheral undifferentiated cells differentiate into glomerulosa cells followed by successive differentiation into fasciculata and ultimately reticulata cells prior to undergoing cell death at the cortical-medullary boundary.
Existence of Undifferentiated Cells
Cells capable of repopulating the adrenocortical steroidogenic zones might be predicted to be undifferentiated and hence pluripotent. The presence of such peripheral undifferentiated cells has been well documented in multiple mammalian species. Most illustrative are studies performed in the artic seal that reveal clusters of rounded large cells with conspicuously large nuclei in the periphery of the adrenal cortex. These cellular aggregates are referred to as blastema - defined as masses of undifferentiated cells capable of undergoing differentiation for growth maintenance of solid organs. The adrenal blastema of the seal reside within the capsule and in perpendicular fibrous cords arising from the capsule that become contiguous with an outer circumferential zone of undifferentiated cells referred to as the zona intermedia (zI) or undifferentiated cell zone (zU) (Vinson 1992; Bragulla et al. 2004). The blastema appear to histologically undergo continuous morphological transition to steroidogenic cells of the zG.
Histological studies in rats have characterized the zI/zU between the zG and the zF (Ogishima et al. 1992; Mitani et al. 1994). BrdU pulse chase and PCNA staining revealed S-phase cells in and around the periphery of the zU, suggesting this population may provide a pool of progenitors that differentiate into the neighboring zones. The variable histological location of clusters and circumferential zones of undifferentiated cells in the adrenal cortices of different species can be attributed to species difference in to the thickness and tortuosity of the glomeruli of the zG that envelop the superior aspect of the blastema (Vinson 1992). The area, encompassing the adrenocortical blastema, zU and zI, previously defined in multiple species, will be referred to hereafter as the subcapsular region. See Figure 2.
Figure 2.
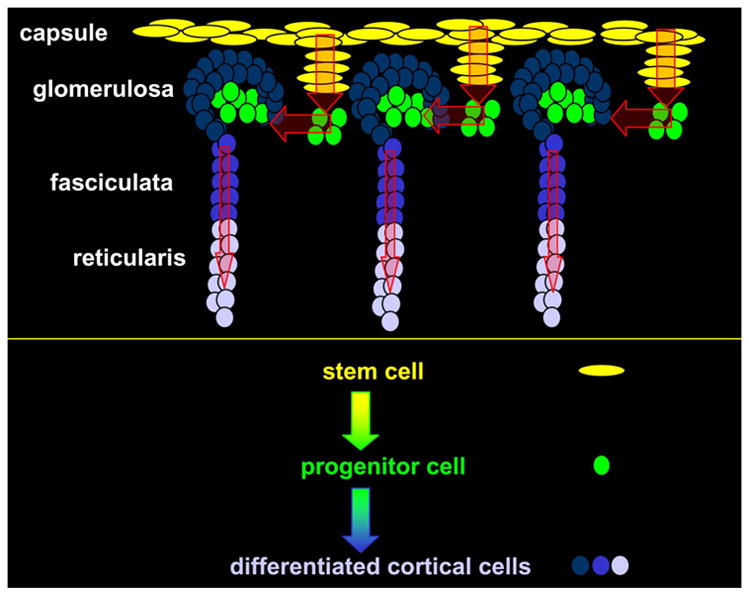
Hypothetical model of stem and progenitor cell centripetal migration and differentiation into steroidogenically competent adrenocortical cells.
Regulation of Mesenchymal Capsule
Molecular identification of the adrenal cortex is defined by the expression of Sf1. Expression of this nuclear receptor is prerequisite for adrenocortical formation and differentiation. In Sf1 deficient mice, adrenocortical development is aborted at the earliest stages of adrenogonadal specification and mice die at birth due to adrenal insufficiency (Luo et al. 1994). In a wildtype mouse, Sf1 expression is evident throughout most of the adrenal gland (from the outer subcapsular region to the cortico-medullary boundary). Importantly, however, Sf1 is not expressed in the mesenchymal capsule, in rare subcapsular cells or in the medulla (Luo et al. 1994; Babu et al. 2002; Beuschlein et al. 2002). As stated earlier, the definitive cortex may arise from the cells of the mesenchymal capsule itself or from rare undifferentiated adrenocortical cells residing within the niche of the capsule. In this scenario, the expression of Sf1 would be predicted to be repressed in the quiescent capsular cells and induced as the cells differentiate into Sf1-positive steroidogenically-negative proliferating progenitor cells.
Many basic-helix-loop-helix (bHLH) transcriptional regulatory proteins have been identified and shown to govern the processes of cellular differentiation and fate determination in several tissues. Pod1/Capsulin/Tcf21 belongs to a sub-family of bHLH proteins that control mesodermal development (Lu et al. 1998; Quaggin et al. 1998). In the developing embryo, Pod1 has been shown to be expressed in the mesenchymal cells at sites of epithelial-mesenchymal interactions within the kidney, lung, intestine, pancreas, gonad and developing fetal adrenal gland (Lu et al. 1998; Quaggin et al. 1998; Tamura et al. 2001; Cui et al. 2004). In the embryonic gonads, Pod1 is expressed in regions from which progenitor cells migrate into the gonads to generate several somatic lineages of the testes. Loss of Pod1 leads to enhanced expression of Sf1, subsequently resulting in premature commitment of progenitor cells to a steroidogenic lineage (Tamura et al. 2001; Cui et al. 2004). In Pod1 deficient testes, the Leydig cell population expands coincident with an increase in Sf1 and Cyp11a1 (a downstream target of Sf1) expression. This is presumably associated with the loss of peritubular myoid cells and pericytes. Therefore, it is proposed that Pod1 represses Sf1 expression in a pluripotent interstitial cell precursor, allowing the differentiation of several interstitial lineages, such as Leydig cells, peritubular myoid cells, and pericytes. Indeed, Pod1 has been shown to specifically inhibit the expression of Sf1 by antagonizing the activity of Usf1 on the proximal E-box of the Sf1 promoter (Cui et al. 2004). Because Pod1 is uniquely expressed in the outer regions of the developing e11.5 mouse cortex (capsule and/or subcapsular region), we hypothesize that the differentiation of Sf1-negative mesenchymal capsule and/or subcapsular cells is mediated by a down-regulation of Pod1 (Lu et al. 1998; Quaggin et al. 1998). Lineage tracing studies with Pod1-Cre mice are in progress in our laboratory to determine if Pod1-positive / Sf1-negative cells give rise to Pod1-negative / Sf1- positive progenitor cells that populate the definitive adrenal cortex (and not the fetal zone/X-zone).
Maintenance of Undifferentiated State of Subcapsular Cells
A subcapsular population of cells (comprised of that are Sf1 positive but steroidogenically negative are proposed to constitute the adrenocortical progenitor cell pool (ie, blastema/zI/zU). These Sf1-positive cells are proposed to remain steroidogenically inactive through the inhibition of Sf1 transactivation by the orphan nuclear receptor, Dax1 (Dosage sensitive sex-reversal, Adrenal hypoplasia congenital locus on the X-chromosome gene 1) (Ito et al. 1997; Crawford et al. 1998; Babu et al. 2002).
Dax1 functions as a coregulatory protein to inhibit transcriptional activity of other nuclear receptors, such as Sf1 (Ito et al. 1997; Crawford et al. 1998; Babu et al. 2002). Dax1 has also been reported to inhibit transcription by binding to DNA hairpins in promoter regions of steroidogenic genes, such as steroidogenic acute regulatory protein (StAR) (Zazopoulos et al. 1997). Lastly, Dax1 participates in tissue fate decisions through dynamic regulation of Gata4/Gata6 transcription factors in adrenals and gonads (Tremblay and Viger 2001; Jimenez et al. 2003). Following stimulation of the adrenal cortex, Dax1 is downregulated, presumably to allow for robust expression of steroidogenic genes (Lalli and Sassone-Corsi 2003).
In recent work, we have shown that Sf1 and Nr3c1 (glucocorticoid receptor, GR) synergistically activate the Dax1 promoter in response to glucocorticoids, while Sf-1 and GR dissociate from the Dax1 promoter in response to ACTH (Gummow et al. 2006). We propose that Dax1 functions to maintain the undifferentiated and proliferative capacity of the adrenocortical subcapsular progenitor cells. Three observations support this hypothesis: 1) Dax1 is restricted expression is restricted to the adrenocortical subcapsular region in males, 2) Dax-1 inhibits SF-1 mediated transcription of steroidogenic genes in cell culture studies and 3) Dax-1 has recently been shown to facilitate maintenance of the undifferentiated state of embryonic stem cells (Mukai et al. 2002; Niakan et al. 2006). While it is known that Dax1 expression is observed throughout the differentiated cortex of females, we hypothesize that Dax1 may have additional roles in females such as the maintenance of the fetal zone/ X- zone. A model is therefore presented whereby subcapsular cells are maintained in an undifferentiated state by an intra-adrenal endocrine feedback mediated by glucocorticoids (generated from the differentiated cells). ACTH stimulation results in an inhibition of Dax1 expression that induces the differentiation and ultimate centripetal turnover of progenitor cells into the zonal compartments of the adrenal cortex. Indeed our work has shown that Dax1 null mice exhibit enhanced steroidogenesis at the expense of a loss of proliferation of subcapsular cell (Babu et al. 2002).
These observations explain in part the variable temporal manifestation of adrenal insufficiency in patients with mutations in DAX1 (X-linked cytomegalic adrenal hypoplasia) (Muscatelli et al. 1994; Lin et al. 2006; Niakan et al. 2006). The loss of DAX1 would be expected to result in an increase of differentiated adrenocortical cells at the expense of the ultimate depletion of the progenitor pool. Different patients would be expected to have intrinsic differences in progenitor cell reserve and hence present with adrenal insufficiency at different times. Current studies are examining this hypothesis in aged Dax1-null mice to determine if the mice eventually develop adrenal hypofunction similar to human patients.
Specificity of Differentiation of Subcapsular Cells
The shared origin of the adrenal and gonad, the existence of pluripotent stem/progenitor cells for steroidogenic lineage in both tissues and the presence of 1) ACTH-driven adrenal rests in patients with long standing untreated congenital adrenal hyperplasia and 2) gonadotropin-driven thecal metaplasia in the adrenal cortex of estrogen-naïve postmenopausal women predict a mechanism that allows the stem/progenitor cells of the each tissue (gonad versus adrenal) to selectively differentiate in response to only the appropriate peptide hormone and not the other (FSH/LH versus ACTH).
The adrenocortical tumors arising in inhibin-α null mice provided a unique opportunity to evaluate this hypothesis. We have presented data that inhibin-α null mice develop adrenocortical blastoma (a neoplasm derived from blastema tissue) that uniquely expresses Cyp19a1, Cyp17a1, and the Lhcgr, in response to chronic LH (Beuschlein et al. 2003). Moreover, recent work in our group has determined that LH and FSH stimulate undifferentiated adrenal subcapsular progenitor cells to develop exclusively into functional ovarian tissue within the adrenal cortex – but only when inhibin is absent (Looyenga and Hammer 2006).
Two events are critical for this ultimate manifestation of ovarian fate in the adrenal cortex of these mice. It appears that the adrenal progenitor cells of wild type adrenal cortex actually have the innate capacity to respond to gonadal-specific differentiation signals (LH and FSH) consistent with the singular origin of the adrenal cortex and gonad – the adrenogonadal primordium. Specifically, LH induces expression of gonadal-restricted Gata4 in the progenitor cells of the adrenal with concomitant loss of adrenal-restricted Gata6, which together are necessary (but not sufficient) to drive adrenocortical progenitor cells to ovarian fate. It is the loss of inhibin and subsequent unopposed Smad3 activation that mediates the expansion of these Gata4-positive cells and ultimate generation of ovarian tissue in the adrenal cortex. In wild type mice, the gonadal-programmed/fated progenitors are prevented from expanding and forming true ovarian tissue through the actions of inhibin (Looyenga and Hammer 2006). When Smad3 is genetically ablated in the context of compound inhibin-α-null / Smad3-null mice, while Gata4-positive cells still reside in the subcapsular progenitor cell region (in response to LH), no expansion occurs indicating the importance of inhibin as a gatekeeper of adrenogonadal fate (unpublished observation).
Summary
This review has detailed experiments that provide support for the existence of undifferentiated pluripotent adrenocortical cells. Enucleation and transplantation studies have revealed the regenerative potential of the adrenal cortex. Although the origin of the regenerating cells remains ambiguous, most data support the contention that the newly generated cells are derived from the periphery of the adrenal cortex. We believe that these differentiated cells arise from capsular and subcapsular cells. It is currently unknown whether fibroblast-like capsular cells themselves undergo differentiation into adrenocortical cells or rare undifferentiated cells, possibly residing within capsule, give arise to differentiated adrenocortical cells. We also do not exclude the possibility that the capsule does not provide undifferentiated cells but rather may act as supporting cells/niche cells for the undifferentiated blastema cells within the capsule and zU/zI that are the bona fide adrenocortical stem cells.
Based on the evidence presented, we propose the following model. Adrenocortical cell repopulation of the cortex in the context of experimental enucleation and physiologic maintenance occurs from the adrenal capsule. The adrenocortical fate of the capsule is repressed through Pod1/capsulin/tcf21 directed inhibition of Sf1. In the presence of a mitogenic signal or through extrinsic cues, the capsule undergoes asymmetric division, resulting in production of another capsular cell and a subcapsular progenitor cell. These subcapsular cells gain Sf1 expression, but are limited in steroidogenic activity due to the Sf1-dependent expression of Dax1. These cells continually proliferate, as evidenced by PCNA and BrdU studies, in order to maintain adrenocortical homeostasis. In response to ACTH, Dax1 is repressed, cells depart centripetally from the subcapsular area and begin to exhibit steroidogenic activity and function as differentiated adrenocortical cells. The specificity of the differentiation program in the adrenal cortex is maintained in part through the actions of inhibin which serves to inhibit the expansion of LH-primed Gata4-positive progenitors destined for ovarian fate.
While the studies described in this review have provided data in support of the presence of adrenal adult “stem” cells, defining undifferentiated adrenocortical cells as bona fide adult stem cells is premature without further in vitro and in vivo characterization utilizing existing tools from tissues with molecular- and cellular-defined stem cells and stem cell niches such as those in the haematopoietic, mammary, dermal, neuronal, and intestinal systems (Fuchs and Segre 2000). For example, flow cytometry may be utilized for isolation of specific cells based on their molecular surface signatures, dye reflux, or fluorescent reporters under the control of well defined genes involved in “stemness”. Moreover, investigation of conserved signaling pathways such as Wnt, Shh, Notch, and TGF-β, that are shown to regulate somatic stem cells in other systems should be conducted (Reya and Clevers 2005). The cre/loxP-conditional knockout technology can be utilized to elucidate the roles of these pathways in adrenocortical lineage determination. Characterizations of adrenal-specific β-Catenin and Apc knock-out mice are among current efforts in our laboratory. Lastly, recent investigations in epigenetic regulation revealing a role of epigenetic regulators, such as the Bmi1 and Ezh2, in maintenance of somatic stem cells suggest that such factors would be important additional targets of inquiry (Park et al. 2003).
Figure 3.
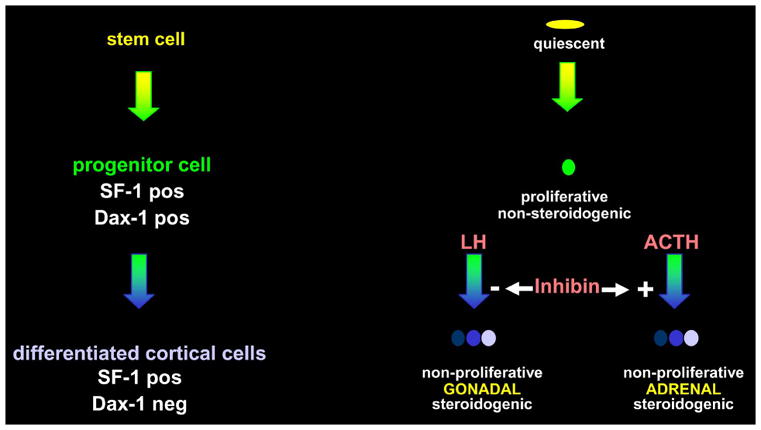
Summary cartoon of molecular events contributing to undifferentiated state of proposed adrenocortical progenitor cells (SF-1 +, Dax-1 +) and the role of inhibin as a gatekeeper of adrenocortical-specific differentiation of these cells (SF-1 +, Dax-1 −) in response to ACTH versus LH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, Hammer GD. Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology. 2002;143(2):665–673. doi: 10.1210/endo.143.2.8658. [DOI] [PubMed] [Google Scholar]
- Baker BL. A comparison of the histologica changes induced by experimental hyperadrenocorticalism and inanition. Recent Prog Hormone Res. 1952;7:331. [Google Scholar]
- Beuschlein F, Looyenga BD, Bleasdale SE, Mutch C, Bavers DL, Parlow AF, Nilson JH, Hammer GD. Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol Cell Biol. 2003;23(11):3951–3964. doi: 10.1128/MCB.23.11.3951-3964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, Hammer GD. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143(8):3122–3135. doi: 10.1210/endo.143.8.8944. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Parviainen H, Porter-Tinge SB, Kiiveri S, Genova E, Rahman N, Huhtaniemi IT, Muglia LJ, Heikinheimo M, Wilson DB. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology. 2003;144(9):4123–4133. doi: 10.1210/en.2003-0126. [DOI] [PubMed] [Google Scholar]
- Bragulla H, Hirschberg RM, Schlotfeldt U, Stede M, Budras KD. On the structure of the adrenal gland of the common seal (Phoca vitulina vitulina) Anat Histol Embryol. 2004;33(5):263–272. doi: 10.1111/j.1439-0264.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- Crawford PA, Dorn C, Sadovsky Y, Milbrandt J. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol. 1998;18(5):2949–2956. doi: 10.1128/mcb.18.5.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE. Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development. 2004;131(16):4095–4105. doi: 10.1242/dev.01266. [DOI] [PubMed] [Google Scholar]
- Else T, Hammer GD. Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab. 2005;16(10):458–468. doi: 10.1016/j.tem.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Ford JK, Young RW. Cell proliferation and displacement in the adrenal cortex of young rats injected with tritiated thymidine. Anat Rec. 1963;146:125–137. doi: 10.1002/ar.1091460206. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA. Stem cells: a new lease on life. Cell. 2000;100(1):143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- Gummow BM, Scheys JO, Cancelli VR, Hammer GD. Reciprocal Regulation of a GR-SF-1 Transcription Complex on the Dax-1 Promoter by Glucocorticoids and ACTH in the Adrenal Cortex. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0461. [DOI] [PubMed] [Google Scholar]
- Iannaccone P, Morley S, Skimina T, Mullins J, Landini G. Cord-like mosaic patches in the adrenal cortex are fractal: implications for growth and development. Faseb J. 2003;17(1):41–43. doi: 10.1096/fj.02-0451fje. [DOI] [PubMed] [Google Scholar]
- Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997;17(3):1476–1483. doi: 10.1128/mcb.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology. 2003;144(10):4285–4288. doi: 10.1210/en.2003-0472. [DOI] [PubMed] [Google Scholar]
- Keene MFL, Hewer EE. The development of the human suprarenal gland. J Anat. 1927;61:302–324. [PMC free article] [PubMed] [Google Scholar]
- Lalli E, Sassone-Corsi P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol. 2003;17(8):1445–1453. doi: 10.1210/me.2003-0159. [DOI] [PubMed] [Google Scholar]
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (SF1/Ad4BP, NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looyenga BD, Hammer GD. Origin and Identity of Adrenocortical Tumors in Inhibin Knockout Mice: Implications for Cellular Plasticity in the Adrenal Cortex. Mol Endocrinol. 2006 doi: 10.1210/me.2006-0182. [DOI] [PubMed] [Google Scholar]
- Lu J, Richardson JA, Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev. 1998;73(1):23–32. doi: 10.1016/s0925-4773(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18(3):378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 1994;135(1):431–438. doi: 10.1210/endo.135.1.8013381. [DOI] [PubMed] [Google Scholar]
- Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, Morohashi K. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells. 2002;7(7):717–729. doi: 10.1046/j.1365-2443.2002.00556.x. [DOI] [PubMed] [Google Scholar]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372(6507):672–676. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- Niakan KK, Davis EC, Clipsham RC, Jiang M, Dehart DB, Sulik KK, McCabe ER. Novel role for the orphan nuclear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol Genet Metab. 2006;88(3):261–271. doi: 10.1016/j.ymgme.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y. Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011 beta in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology. 1992;130(5):2971–2977. doi: 10.1210/endo.130.5.1572304. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Perrone RD, Bengele HH, Alexander EA. Sodium retention after adrenal enucleation. Am J Physiol. 1986;250(1 Pt 1):E1–12. doi: 10.1152/ajpendo.1986.250.1.E1. [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Ferreira J, Vendeira P, Magalhaes MC, Vinson GP. Proliferation of capsular stem cells induced by ACTH in the rat adrenal cortex. Endocr Res. 2002;28(4):683–691. doi: 10.1081/erc-120016987. [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Vanden Heuvel GB, Igarashi P. Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev. 1998;71(1–2):37–48. doi: 10.1016/s0925-4773(97)00201-3. [DOI] [PubMed] [Google Scholar]
- Race G, Green RF. Zonation and regeneration of the adrenal cortex of the rat. A M A Arch Path. 1955;59:578. [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Salmon TN, Zwemer RL. A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. Anat Rec. 1941;80(4):421–429. [Google Scholar]
- Skelton FR. Adrenal regeneration and adrenal-regeneration hypertension. Physiol Rev. 1959;39(1):162–182. doi: 10.1152/physrev.1959.39.1.162. [DOI] [PubMed] [Google Scholar]
- Sucheston ME, Cannon MS. Development of zonular patterns in the human adrenal gland. J Morphol. 1968;126(4):477–491. doi: 10.1002/jmor.1051260408. [DOI] [PubMed] [Google Scholar]
- Tamura M, Kanno Y, Chuma S, Saito T, Nakatsuji N. Pod-1/Capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech Dev. 2001;102(1–2):135–144. doi: 10.1016/s0925-4773(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Thomas M, Northrup SR, Hornsby PJ. Adrenocortical tissue formed by transplantation of normal clones of bovine adrenocortical cells in scid mice replaces the essential functions of the animals’ adrenal glands. Nat Med. 1997;3(9):978–983. doi: 10.1038/nm0997-978. [DOI] [PubMed] [Google Scholar]
- Thomas M, Yang L, Hornsby PJ. Formation of functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat Biotechnol. 2000;18(1):39–42. doi: 10.1038/71894. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol Reprod. 2001;64(4):1191–1199. doi: 10.1095/biolreprod64.4.1191. [DOI] [PubMed] [Google Scholar]
- Uotila UU. The early embryological development of the fetal and permanent adrenal cortex in man. The Anatomical Record. 1940;76(2):183–203. [Google Scholar]
- Vinson GP, Whitehouse B, Hinson J. The Adrenal Cortex. Prentice Hall Inc.; 1992. [Google Scholar]
- Weinberg WC, Howard JC, Iannaccone PM. Histological demonstration of mosaicism in a series of chimeric rats produced between congenic strains. Science. 1985;227(4686):524–527. doi: 10.1126/science.3966159. [DOI] [PubMed] [Google Scholar]
- Wright NA, Voncina D, Morley AR. An attempt to demonstrate cell migration from the zona glomerulosa in the prepubertal male rat adrenal cortex. J Endocrinol. 1973;59(3):451–459. doi: 10.1677/joe.0.0590451. [DOI] [PubMed] [Google Scholar]
- Zajicek G, Ariel I, Arber N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol. 1986;111(3):477–482. doi: 10.1677/joe.0.1110477. [DOI] [PubMed] [Google Scholar]
- Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390(6657):311–315. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwemer RL, Wotton RM, Norkus MG. A study of corticoadrenal cells. Anat Rec. 1938;72(2):249–263. [Google Scholar]


