Abstract
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease with over 200 million individuals infected worldwide. The vast majority of acutely infected humans develop chronic infection, which is characterized by attenuated antiviral T-cell responses. The mechanisms leading to such attenuation/suppression are poorly understood. It has been proposed that dysfunction of antigen-presenting cells (APC) may underlie the downregulation of antiviral immune responses. However, studies using bulk or in vitro-derived APC populations have resulted in conflicting reports. In this study, we evaluated the functional and immunophenotypic features of ex vivo-purified dendritic cell (DC) subsets during chronic HCV infection. We found that plasmacytoid DC (PDC) from HCV-infected patients (HCV-PDC) showed a striking deficit in IFN-α production in response to CpG stimulation. stimulation. In addition, we found that myeloid DC (MDC) from these patients showed a diminished capacity to induce a mixed lymphocyte response (MLR), correlating with lower levels of HLA-DR and CD86 expression and higher IL-10 production in response to poly-IC stimulation. In contrast, HCV-PDC showed increased ability to stimulate an MLR. Of note, within the HCV-PDC preparation, we noted a distinctly expanded DC subset that expressed some markers of MDC, but showed significantly lower HLA-DR and CD86 expression, suggesting an expansion of DC at an immature/intermediate stage of differentiation. Our studies demonstrate distinct and contrasting dysfunctional features in DC subsets and underscore the importance of evaluating APC subpopulations separately.
INTRODUCTION
Hepatitis C virus (HCV) infection is a major health problem worldwide and is a leading cause of chronic liver disease, associated with significant morbidity and mortality [1]. Over 80% of acutely infected individuals are unable to clear the virus, resulting in chronic infection and increased potential to develop cirrhosis, end-stage liver disease and hepatocellular carcinoma [2]. It is well documented that a strong and sustained antiviral T-cell response is associated with a self-limited course after acute infection [3; 4; 5]. In contrast, chronic HCV infection is characterized by attenuated CD4+ and CD8+ HCV-specific T-cell responses [5; 6; 7; 8; 9]. Successful therapy of chronic infection also appears to correlate with higher antiviral T-cell responses [9; 10; 11].
The causes of attenuation of immune responses to HCV during chronic infection are poorly understood, as are mechanisms of therapeutic enhancement. It has been proposed that suboptimal or failed antigen presentation during chronic HCV infection may be responsible for the inhibition of antiviral T-cell responses. Dendritic cells (DC) are the most potent professional antigen presenting cells (APC) that play a pivotal role in the initiation and maintenance of CD4+ and CD8+ T-cell responses. There are two distinct subpopulations of DC: the myeloid DC (MDC) and the plasmacytoid DC (PDC). MDC, derived from a myeloid bone marrow precursor, function primarily in uptake, processing and antigen presentation while PDC, presumably derived from a lymphoid precursor, are the main producers of type I interferons and are considered the natural interferon-producing cells [12]. There are conflicting reports in the literature as to the frequency and functional capacity of both MDC and PDC in HCV infection. While some investigators have found a decrease in functional capacity of MDC to elicit an effective allogeneic mixed lymphocyte reaction (MLR) and PDC to secrete interferon-alpha, others have not [13; 14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26]. The vast majority of prior studies have assessed the functional capacity of APC within bulk PBMC cultures or in vitro-derived DC. Very few studies have assessed the immunophenotypic and functional capacity of mature MDC and PDC, purified ex vivo from chronic HCV patients. MDC and PDC have distinct functional attributes that may be differentially affected in vivo during chronic HCV infection, making it important to evaluate these subsets separately. Thus, in this study, we directly analyzed the immunophenotypic and functional features of ex vivo-purified MDC and PDC subsets, comprising ≤ 0.5% of PBMC. We show here that MDC and PDC exhibit differential dysfunctional features in patients with chronic HCV infection. These studies may explain the conflicting results obtained from the evaluation of bulk APC populations and provide new insights into the subtle immune dysfunction that underlies chronic HCV infection.
MATERIALS AND METHODS
Subjects
Twelve treatment-naïve patients with chronic HCV infection were recruited for this study. The patient characteristics are listed in Table 1. Informed consent was obtained from all patients for the blood draw, as approved by the UT Southwestern IRB. Twelve healthy blood donors served as study controls.
Table 1.
Subject Characteristics
| Characteristics | HCV Patients (n = 12) | Healthy Subjects (n = 12) |
|---|---|---|
| Age (yr); Mean (Range) | 49 (25–62) | 42 (21–55) |
| Sex (M/F) | 6/6 | 8/4 |
| AST (IU/L); Mean (Range) | 49 (14–106) | N/A |
| ALT (IU/L); Mean (Range) | 42 (15–117) | N/A |
| HCV Viral Load (KIU/ml) Mean (Range) | 2201 (232–6250) | N/A |
| Histology: Grade/Stage; per Batts and Ludwig[59]; Range | I–II/I–III | N/A |
Isolation of DC subsets and CD3+ T-cells
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation method using Ficoll-Hypaque Plus (GE Healthcare) and were cryopreserved at 10 X 106/ml in 10% DMSO and 90% FBS (Hyclone, Utah) until use. On the day of experiment, MDC and PDC subsets were isolated from thawed PBMC using isolation kits from Miltenyi Biotech, Auburn CA (BDCA-1 positive cell isolation kit for myeloid DC and BDCA-4 positive cell isolation kit for plasmacytoid DC), according to the manufacturer’s instructions (that included B-cell depletion prior to MDC isolation). Of note, in initial experiments, DC subsets purified from fresh vs. cryopreserved PBMC obtained from three healthy-HCV donor pairs showed no significant differences in viability, immunophenotype or functional capacity, as assessed in these assays.
Third-party T-cells for MLR experiments were isolated from fresh PBMC of a single healthy donor using a commercial kit (CD3+ T-cell isolation kit; R&D, MN), yielding >98% purity.
MDC and PDC cultures
PDC and MDC were isolated in parallel from a HCV and healthy donor pair in each experimental setup and were cultured separately at 2 x 105 cells/ml in 0.2 ml of culture media in 96-well round bottom plates. Culture media used in all experiments was RPMI 1640 media (Mediatech, VA) supplemented with 5% heat-inactivated Human AB serum (Gemini Bioproducts), L-glutamine (2 mM), penicillin (50 U/ml), and streptomycin (50 U/ml; Invitrogen). MDC and PDC were cultured for 2 days in media alone, or with either poly-IC (50 μg/ml) or CpG (ODN-2216; 6 μg/ml), respectively (Invivogen, San Diego, CA). Supernatants were harvested at 48 hours and frozen until evaluation.
Flow cytometric evaluation of DC
Flow cytometric staining, data acquisition and analysis were performed, as described [27]. The following reagents were used: allophycocyanin (APC)-anti-CD11c, fluorescein isothiocyanate (FITC)-anti-HLA-DR, phycoerythrin (PE)-anti-CD123, PeCy5-anti-CD86 (BD Biosciences, San Jose, CA). Staining with isotypic controls was used to determine cutoffs. In addition, antibodies against CD3, CD19, CD20 and CD14 were used in the detailed evaluation of DC subsets.
Allogeneic mixed lymphocyte reaction (MLR)
After optimization of culture conditions for allogeneic MLR (not shown), purified T-cells (1 x 105) from a single healthy donor were cultured in triplicate with MDC or PDC (0.16 x 105) in 0.2 ml of media in 96-well round bottom plates for 6 days. T-cells cultured alone or with autologous APC served as a negative controls and showed negligible thymidine incorporation (not shown). During the last 16 hr of incubation, the cultures were pulsed with 2.0 μCi/well of 3H-thymidine (GA Healthcare). On day 6, the cells were harvested and the thymidine incorporation (proliferation) was quantified as counts per minute (CPM), as described previously [27].
ELISA for cytokines
Supernatants from MDC and PDC cultures were used to quantify the secretion of IL-12 p70 and IL-10 (for MDC) or IFN-α (PDC). Standard ELISA assays were performed according to manufacturer instructions (IL-12 p70, IL-10; R& D systems, MN and IFN-alpha; PBL Biomedical Labs).
Statistical analysis
Differences in various readouts between the two cohorts were determined by two-tailed student’s t-tests. P values <0.05 were considered to be statistically significant.
RESULTS
MDC from HCV-infected patients show lower allostimulatory capacity and lower expression of HLA-DR and CD86, compared to healthy MDC
First, we obtained ex vivo-purified populations of MDC by using anti-BDCA-1-based magnetic bead sorting. MDC preparations from healthy subjects and HCV patients (HCV-MDC) exhibited the expected CD11cbright +/CD123moderate + to − immunophenotype that characterizes circulating mature MDC, with >90% purity (Fig. 1).
Figure 1. Comparable immunophenotype and purity of MDC obtained from HCV patients and healthy subjects.
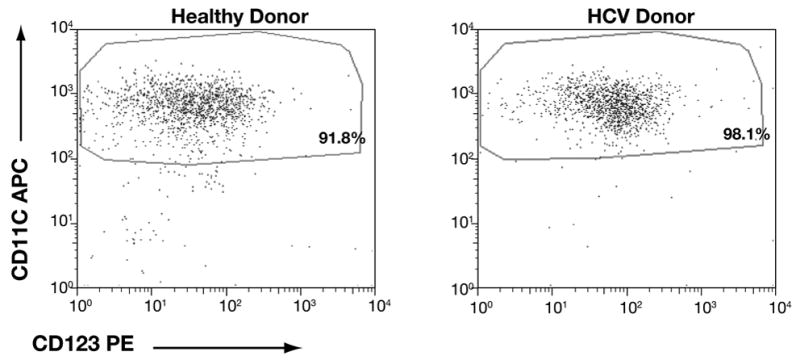
These dotplots represent bead-purified MDC from a healthy subject (top panel) and an untreated chronic HCV patient (bottom panel). These preparations showed the expected CD11cbright +/CD123moderate + to − phenotype with >90% purity. This is representative of 10 subject pairs, evaluated in parallel.
These MDC were used in an allogeneic MLR to evaluate their overall capacity as APC. One HCV-healthy subject pair was evaluated at each experimental setup. In addition, we used T-cell responder cells from the same donor across multiple experiments to allow for comparison of APC capacity across the board. We observed that MDC from HCV-infected patients demonstrated a significantly lower capacity to elicit an MLR, compared to those from healthy donors (Fig. 2). While there was a clear trend toward reduced MLR in the majority of paired specimens, it is also important to note that the APC function was not completely abrogated in ex vivo-purified HCV-MDC.
Figure 2. Lower allostimulatory capacity of MDC from HCV-infected patients, compared to healthy donors.
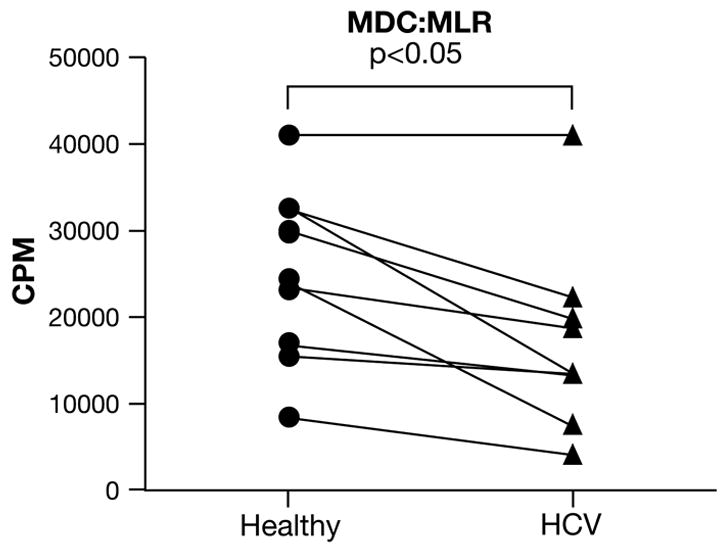
Purified MDC from either healthy donors (circles) or from HCV-infected patients (triangle) were used to stimulate purified allogeneic T-cells from a healthy subject (third party). Cultures were pulsed with 3H-thymidine on day 5 and harvested and counted on day 6. One healthy subject and one HCV patient were always evaluated as a pair (as indicated by the lines, n=8 pairs) and exposed to the same third party responder T-cells in all experiments. The data represent a stimulator:responder ratio of 1:6. Similar pattern was observed at other ratios. Ex vivo-purified MDC from HCV subjects showed a lower capacity to induce an MLR (17055 ± 3557 CPM), compared to healthy MDC (24970 ± 3394 CPM; p<0.05).
We also evaluated the expression of the MHC Class II molecule, HLA-DR, and the co-stimulatory molecule, CD86, as these molecules contribute to the APC function of MDC. We found that both HLA-DR and CD86 expression was significantly lower on the surface of HCV-MDC, compared to healthy MDC (Fig. 3), correlating with their decreased ability to induce an MLR. This pattern of lower HLA-DR and CD86 was maintained in MDC that were cultured either in media alone or stimulated with the toll-like receptor (TLR)-3 ligand, poly-IC (not shown).
Figure 3. Slightly lower HLA-DR and CD86 expression on HCV-MDC.
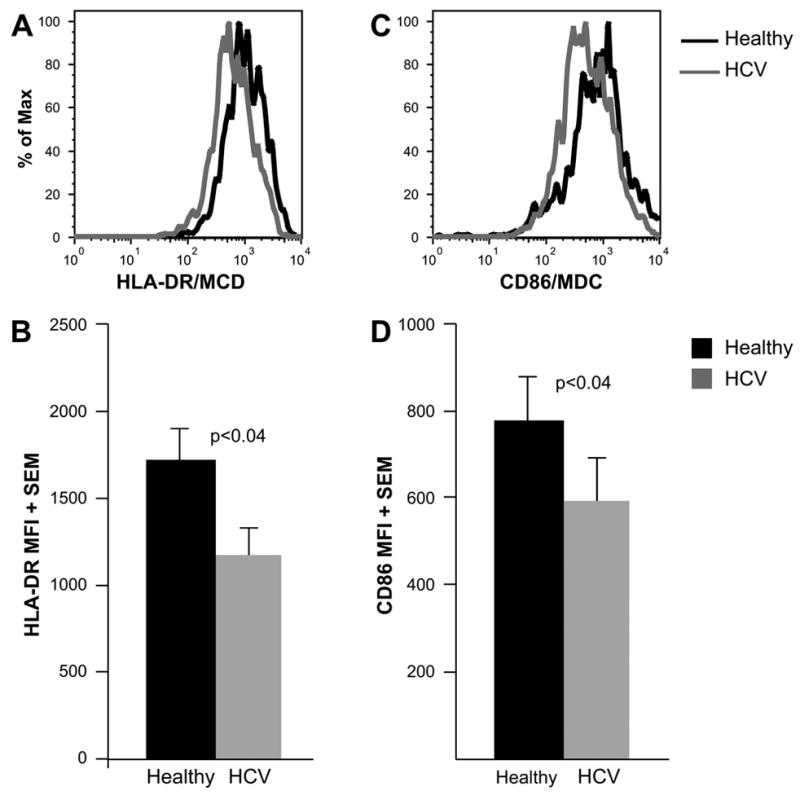
The histograms (A and C) depict representative examples of HLA-DR (A) and CD86 (C) expression on healthy and HCV-MDC. The graphs (B and D) represent the average mean fluorescence intensity (MFI) of HLA-DR (B) and CD86 (D) expression on poly-IC-stimulated MDC from 10 healthy-HCV pairs. MDC rested in media alone also showed a similar trend (data not shown). MDC from HCV patients showed slightly but consistently lower HLA-DR and CD86 expression compared to healthy MDC (p<0.04).
High IL-10 and low IL-12 production by poly-IC-stimulated HCV-MDC
It has been proposed that DC from HCV-infected individuals may promote a Th2 response, rather than an effector Th1 response owing to their cytokine profile [22; 28; 29]. We thus measured the production of IL-12 and IL-10 in 2-day supernatants from poly-IC-stimulated MDC cultures. Interestingly, we observed low-level constitutive IL-10 production from HCV-MDC (Fig. 4A). Moreover, poly-IC-stimulated HCV-MDC produced significantly greater amounts of IL-10, compared to healthy MDC. In contrast, HCV-MDC cultures showed a tendency toward lower production of the Th1-promoting cytokine, IL-12, although this did not reach statistical significance (Fig. 4B). Overall, these results suggest a tendency toward promoting Th2 or regulatory T-cell responses by MDC in HCV-infected patients.
Figure 4. Production of IL-10 and IL-12 by MDC from healthy subjects and HCV patients.
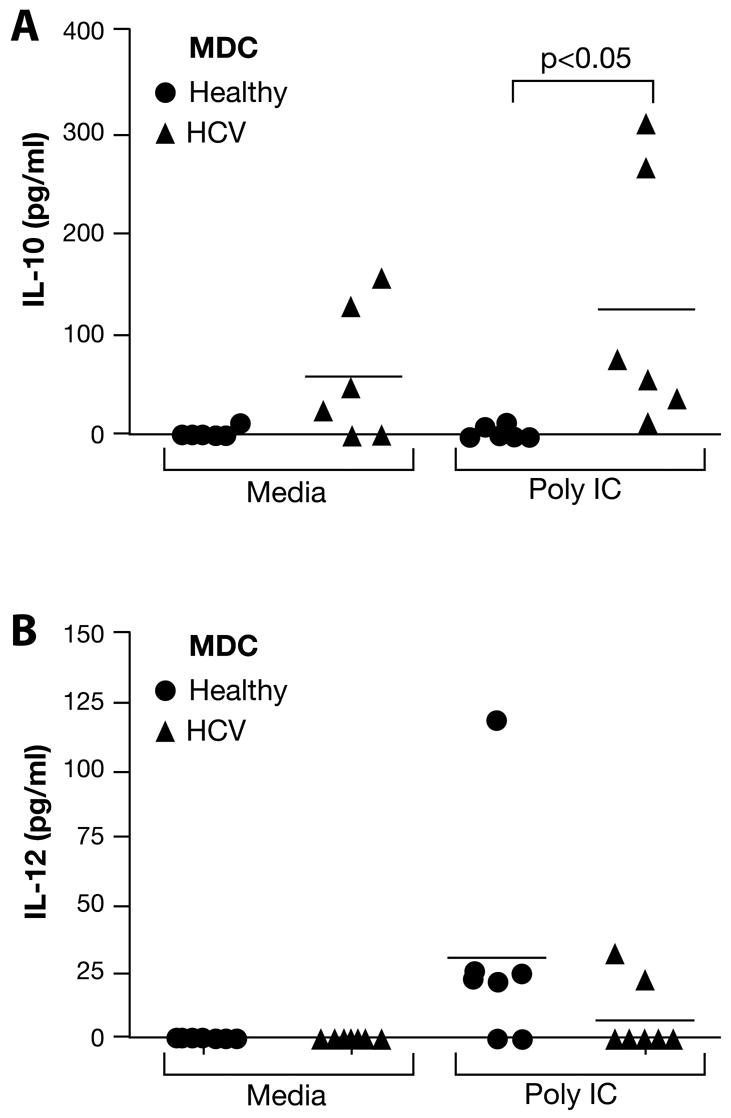
Purified MDC were cultured for 2 days in media alone or with Poly-IC (50 μg/ml). The levels of IL-10 (A) or IL-12 (B) were measured in 2-day supernatants by ELISA. In contrast to healthy MDC, HCV-MDC showed constitutive IL-10 production in media alone. The level of IL-10 produced by HCV-MDC in response to Poly-IC was significantly higher than that produced by healthy MDC (p<0.05). In contrast, IL-12 production by healthy MDC tended to be higher than HCV-MDC, although the difference was not statistically significant (p<0.09).
PDC from HCV-infected patients show a striking deficit in their ability to secrete IFN-α
To delineate the function of PDC in HCV-infected individuals, we used the BDCA-4 microbead-based isolation kit for ex vivo purification of circulating PDC. As expected, the PDC from healthy subjects showed one predominant cluster of cells with the typical CD123bright+/CD11c− immunophenotype that characterizes mature circulating PDC (Fig. 5A). However, PDC preparations from HCV patients (HCV-PDC) showed another distinct subset that exhibited a CD11c+/CD123dim+ phenotype. Of note, this subset was also observed in healthy PDC preparations, but it was significantly expanded in HCV-PDC preparations (6.4% ± 2.9% vs. 31.6% ± 5.4%; p<0.01) (Fig. 5A and 5B).
Figure 5. A significantly expanded subset of DC in PDC preparations from HCV-infected patients.
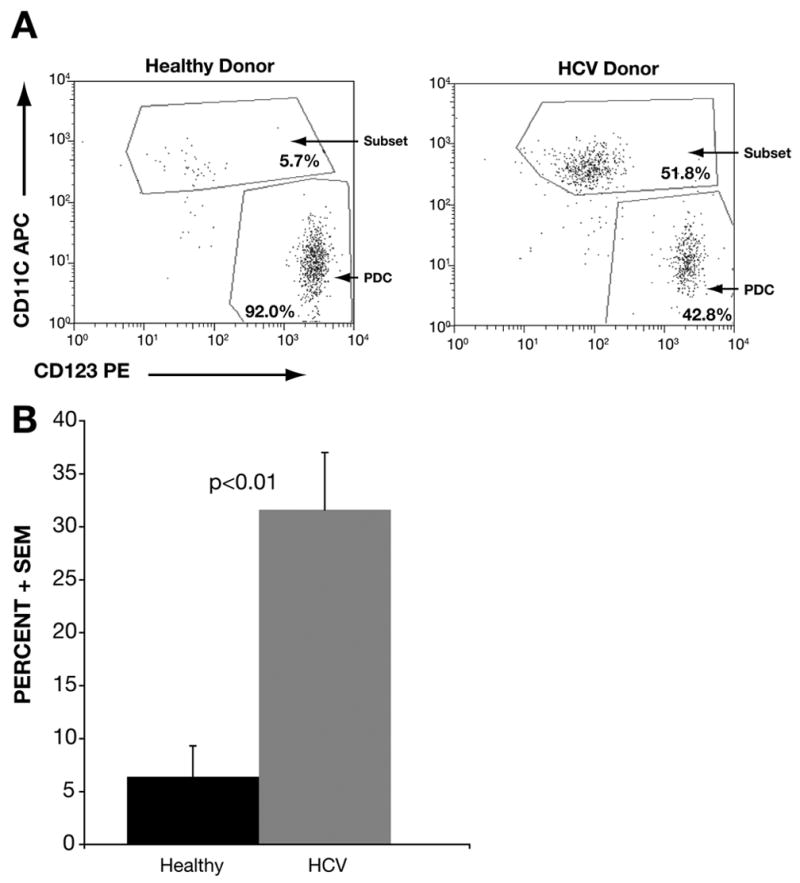
Panel A shows representative dot-plots of bead-purified PDC preparations from a healthy subject (top panel) and an untreated HCV-infected patient (bottom panel). PDC were isolated from PBMC using a Miltenyi Biotech microbead kit (based on BDCA-4 expression). As expected, healthy PDC showed >90% purity, with a predominant population showing the characteristic CD123bright +/CD11c− phenotype. However, the purified PDC also showed a distinct subpopulation showing a CD11c+/CD123moderate to dim + phenotype, which was greatly expanded in HCV-PDC preparation. Panel B shows an analysis of 10 healthy-HCV subject pairs. The bars represent the mean percentage (+ SEM) of this DC-subset in PDC preparations from healthy subjects (6.4% ± 2.9%) vs. HCV patients (31.6% ± 5.4%), showing a significant expansion of this subset in HCV patients (p<0.01).
As separation of MDC and PDC were sequential steps in our experiments (MDC before PDC), it is unlikely that this subset represents contaminating MDC. When adequate cell numbers were available, we also confirmed this finding in the case of separately performed preparations (non-sequential), or in reverse sequence. These cells also exhibited forward and orthogonal light scatter properties that were distinct from lymphocytes and similar to other DC preparations, in that they were larger and slightly more complex cells. We further confirmed that these were not contaminating lymphocytes or monocytes, as they were negative for CD3, CD19, CD20 and CD14 (data not shown). Moreover, as discussed later, these cells uniformly expressed HLA-DR, but at levels distinct from mature MDC. Thus, rather than representing contaminating mature MDC or lymphocyte subsets, the overall features suggested an expanded population of DC with a relatively immature state of differentiation.
While PDC can serve as APC, their major function in the antiviral response appears to be the production of type I interferons. They are the main producers of IFN-α during a viral infection. Hence, we tested the ability of HCV-PDC to produce IFN-α in response to stimulation with the TLR-9 agonist CpG. We found that the ability to produce IFN-α was dramatically abrogated in HCV-PDC (Fig. 6). As explained above, the HCV-PDC preparations contained an expanded subset of immature DC with intermediate immunophenotypic features (at a frequency of 31.6% ± 5.4%). Thus, one could argue that the lower production of IFN-α reflected the lower numbers of mature PDC in these cultures. However, the difference in IFN-α was so profound that it could not be explained by lower cell numbers alone (Fig. 5). These results suggest that, in HCV-infected patients, even mature PDC exhibit an IFN-α deficit on a per cell basis.
Figure 6. Dramatically deficient interferon-α production by HCV-PDC.
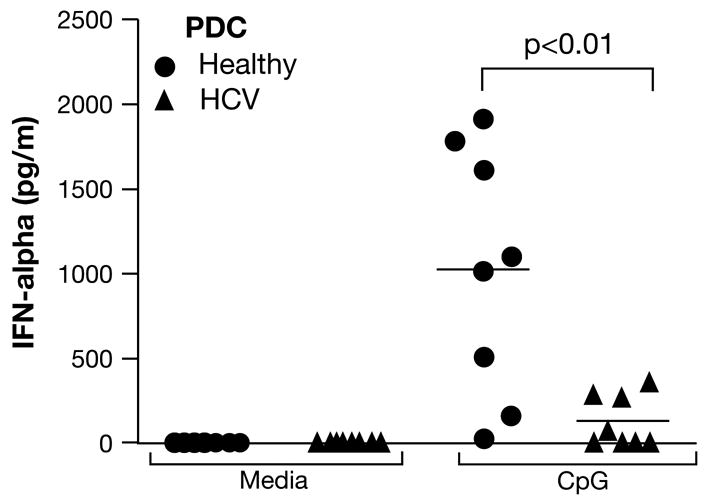
PDC were cultured for 2 days in media alone, or with CpG (6 μg/ml). IFN-α levels in supernatants were quantified by ELISA. HCV-PDC (triangles) showed a remarkably reduced capacity to produce IFN-α in response to CpG, compared to healthy PDC (circles; p<0.01). PDC produced negligible IFN-α in media alone.
PDC from HCV-infected patients show higher allostimulatory capacity, compared to healthy PDC
While MDC are 10–50 times more efficient as APC, compared to PDC, PDC have been shown to be capable of antigen presentation. Thus, in addition to IFN-α production, we also evaluated the APC functions of PDC. Interestingly, HCV-PDC stimulated a significantly greater MLR compared to healthy PDC. The difference in the MLR responses were small enough to be explained, in part, by the expanded subset of DC, which may have some MDC-like features (see below). However, this also suggests that distinct subsets of DC from HCV-infected patients show differentially affected APC ability. Thus, experiments using bulk PBMC preparations may result in one population of APC negating the dysfunctional features of another in in vitro assay systems.
Similar to our evaluation of MDC, we also quantified HLA-DR and CD86 expression on PDC. As we found two distinct subsets, we evaluated them separately for these markers. Mature PDC from both healthy subjects and HCV-infected patients expressed comparable levels of HLA-DR and CD86 (Fig. 8). As expected, these levels were significantly lower than those of MDC (Fig. 3), correlating with a lower capacity of PDC to induce an MLR.
Figure 8. Expression of HLA-DR and CD86 on PDC preparations from healthy subjects and HCV-infected patients.
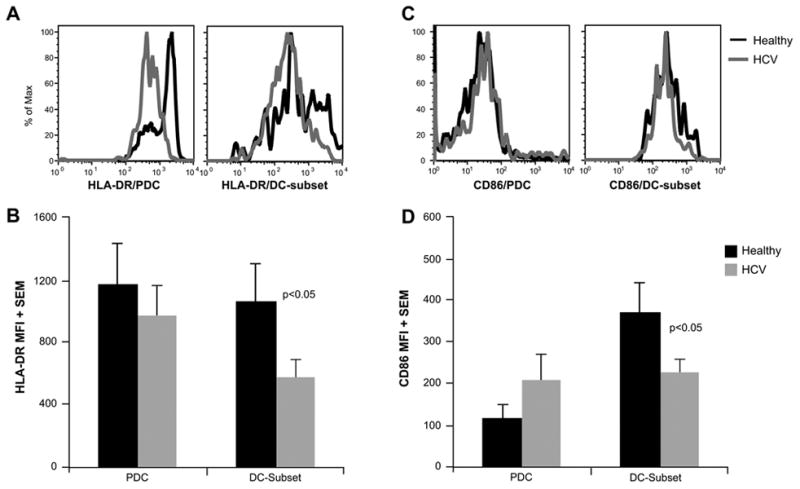
The histograms (A and C) depict representative examples of HLA-DR (A) and CD86 (C) expression on PDC preparations from a healthy subject and an HCV patient. The mature PDC and immature DC subset were evaluated separately (see text and Figure 5). The graphs represent the average mean fluorescence intensity (MFI) of HLA-DR (B) and CD86 (D) expression on CpG-stimulated PDC preparations from 10 healthy-HCV pairs. PDC rested in media alone also showed a similar pattern (data not shown). The mature PDC from healthy subjects or HCV patients showed comparable HLA-DR and CD86 expression. In contrast, the immature DC subset from HCV patients showed lower HLA-DR and CD86 expression. Of note, the HLA-DR and CD86 expression on this subset were significantly lower than that of MDC (Figure 3). CD86 expression on this subset was higher than that of mature PDC (p<0.05).
HLA-DR and CD86 expression on the expanded DC subset was also significantly lower than that of MDC (Figs. 3 and 8), indicating that these cells did not represent contaminating MDC, but rather may represent DC at an intermediate/immature state of differentiation. Interestingly, this subset of DC showed slightly higher CD86 expression compared to mature PDC and this may have contributed to enhanced MLR stimulated by these PDC preparations, as discussed above. Of note, both HLA-DR and CD86 expression was significantly lower in this subset of cells obtained from HCV patients, compared to those from healthy subjects. Thus, not only were these cells significantly expanded during HCV infection, they also showed lower expression of APC function-related molecules, which may account for overall deficit in APC function in combined APC populations.
DISCUSSION
Patients with chronic HCV infection show attenuated HCV-specific CD4+ and CD8+ T-cell responses. The causes for such attenuation are unclear and may include failure of antigen presentation, T-cell exhaustion and dysfunction, viral mutations, intra-hepatic modulation or increased regulatory T-cell function [4; 13; 30; 31; 32; 33; 34; 35; 36; 37; 38; 39]. Studies evaluating the function of APC during chronic HCV infection have resulted in conflicting reports [13; 14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26]. However, the majority of these studies has used either bulk APC or in vitro-derived DC cultures. Evaluation of bulk PBMC may be easily affected by minor variations in the size of small DC populations. Moreover, it is also possible that differentially affected APC subsets may negate each other in the total effect of bulk PBMC. Studies using in vitro-derived DC simply test the ability of monocytes to be differentiated into full-fledged DC. This readout is quite distinct from the state of ex vivo-purified individual APC subsets directly from the patients. Thus, the focus of our study was to specifically evaluate ex vivo-purified DC subsets (MDC and PDC) from HCV-infected individuals with regard to immunophenotypic and functional features. As each of these subsets make up a small percentage of PBMC (~0.3–0.8%), we restricted the number of readouts to the evaluation of APC function in an MLR, the immunophenotypic attributes and the production of relevant cytokines in response to relevant stimuli (poly-IC for MDC and CpG for PDC).
In our study, the most pronounced defect within mature ex-vivo purified DC populations was the diminished capacity of PDC from HCV-infected patients to produce IFN-α in response to the TLR-9 agonist, CpG. Several prior studies, predominantly based on bulk PBMC cultures, corroborate our findings and report a downregulation of IFN-α production, albeit leaving it unclear whether this is related to the lower number of PDC in HCV patients or to an intrinsic defect [17; 40]. In our study, despite the relatively lower percentage of mature PDC in the BDCA-4-derived subset from HCV patients, it is clear that the reduced IFN-α production is reflective of an intrinsic defect of mature PDC from HCV-infected individuals. It is well accepted that HCV infection can result in the blockade of IFN-α responses through interference with several intracellular signaling pathways in hepatocytic cultures. However, the presence of direct HCV infection of immune cells is a controversial area where some investigators report evidence of productive infection while others refute this claim [15; 41; 42; 43; 44; 45; 46; 47]. Interestingly, infection of DC with impairment of maturation and function is a strategy utilized by various viruses to avoid targeting by the immune system. DC-SIGN, a receptor on DC, has recently been found to be of importance in the uptake and processing of HCV, through interaction with the envelope proteins E1 and E2 [48]. DC-SIGN also mediates the uptake on DC of HIV, Ebola, CMV, Mycobacterium tuberculosis and other pathogens [48]. The presence of both positive and negative strands of HCV in monocyte-derived DC, as well as, in circulating mature DC lends support to the theory that HCV is capable of infecting and replicating within DC and thus, may directly modulate differentiation and function of DC [15; 28]. DC transfected in vitro with HCV proteins or vectors encoding HCV genes, have lower stimulatory capacity, related to the lack of maturation following activation with TNF-alpha or CD40L [49]. In the same manner, core-expressing macrophages produce less IL-12 after stimulation. Preliminary studies from our laboratory have not revealed evidence for replicative infection of ex vivo-purified DC (not shown). Thus, it is unclear whether PDC dysfunction reflects a direct or indirect effect of HCV infection. Even the presence of adequate numbers of positive strand RNA inside cells may be potentially sufficient to produce viral proteins that interfere with cell function, without the need for replication. Moreover, it has been reported that exogenous HCV proteins may have several effects on DC. They can act through a complement receptor inhibiting IL-12 production. Furthermore, they can interact with monocytes/macrophages through TLR2, inducing the production of IL-10. IL-10 inhibits IL-12 production by MDC and IFN-α by PDC. Furthermore, exogenous HCV proteins have been shown to directly inhibit DC differentiation [26]. Overall, the in vivo mechanisms of this PDC dysfunction remain unclear and need future investigation.
Another novel and interesting difference between healthy subjects and HCV patients, demonstrated by the current study, is the presence of an expanded population of DC with unusual immunophenotypic features in BDCA-4-bead-sorted PDC preparations. While similar cells were also observed in preparations from healthy PBMC, HCV patients showed significant expansion of this subset. These cells did not represent contaminating MDC; rather, they expressed a phenotype intermediate between MDC and PDC and showed lower expression of HLA-DR and CD86, compared to MDC. Thus, there appears to be an expansion of circulating immature/intermediate DC in HCV-infected subjects, which may be a result of in vivo stimulation of a subset of DC into expressing this unusual phenotype, as suggested in prior in vitro studies [50], representing the plasticity between MDC and PDC populations that has been described in other viral infections [51].
We also show that HCV-PDC preparations have an enhanced ability to stimulate an in vitro MLR, albeit lower than that of MDC from either healthy or HCV donors. The presence of the expanded immature/intermediate PDC-subset in HCV patients, with some MDC-like features may explain the subtle enhancement of MLR responses induced by HCV-PDC preparations, correlating with a significantly higher level of expression of the HLA-DR and CD86 on the immature/intermediate DC subset, compared to mature PDC. An admixture of such cells could affect the readouts in the functional evaluation of bulk PBMC populations.
Our study also revealed a functional defect in the ex-vivo purified mature MDC obtained from chronic HCV patients. We demonstrate that HCV-MDC stimulate a less robust MLR, compared to healthy MDC, correlating with a lower level of HLA-DR and CD86 expression. These findings are supported by prior studies [15; 29; 52]. Moreover, HCV-MDC produced significantly more IL-10 and tended to produce lower IL-12 following in vitro stimulation. This profile appears more supportive of a Th2 (rather than Th1) response, as suggested by prior studies [29; 52]. However, it is important to note that this functional “deficit” in HCV-MDC was mild in magnitude and did not reflect completely abrogated APC functionality. With regard to clinical presentation, patients with chronic HCV infection are not overtly “immunosuppressed” and are likely capable of initiating other immune responses. However, they do exhibit subtle immunologic deviation in terms of the immunophenotypic features of antiviral responses to ubiquitous viruses (CMV, EBV) or their ability to mount a robust response to vaccination [53; 54; 55]. It stands to reason that there would be only subtle functional effects on different APC subsets. Thus, the expansion of relatively immature DC, in combination with the mild APC defect in mature MDC, may explain the relatively mild “immune deficit” seen in these patients. Also, the opposite effects of distinct APC subsets on MLR responses may explain the conflicting results using bulk PBMC as APC. In recent studies, we have observed that B cell populations from HCV patients induce greater MLR responses (not shown). Recent studies have also proposed a hyper-activated state for B-cells during HCV infection [56; 57; 58], further underscoring the necessity to evaluate each APC population separately. These observations may also explain the relatively HCV-specific nature of the immune attenuation, as one would expect the greatest attenuation of ongoing responses with ongoing T-cell:APC interactions.
Figure 7. Higher allostimulatory capacity of PDC from HCV-infected patients, compared to healthy donors.
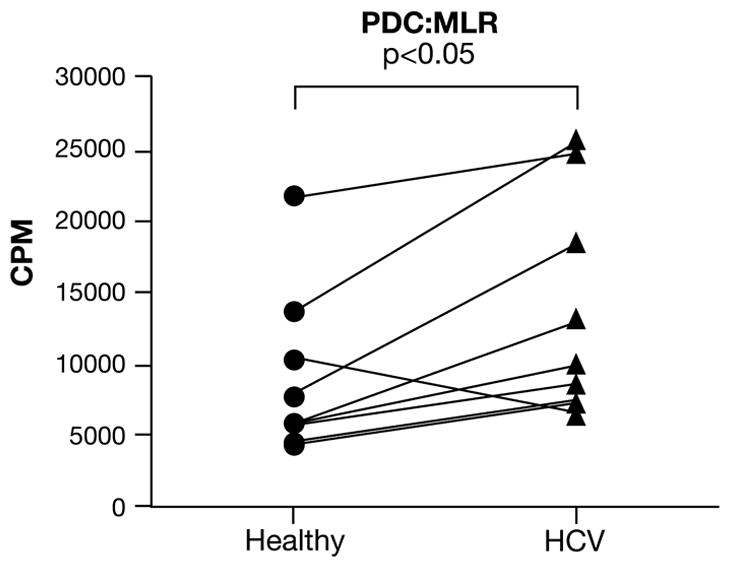
PDC preparations from either healthy donors (circle) or HCV-infected patients (triangle) were used as stimulators with purified allogeneic healthy T-cells. One healthy subject and one HCV patient were always evaluated as a pair (as indicated by the lines, n=8 pairs) and exposed to the same third party responder T-cells in all experiments. The data represent a stimulator:responder ratio of 1:6. Similar pattern was observed at other ratios. PDC from HCV subjects showed a significantly elevated capacity to induce an MLR (Healthy; 8911 ± 1917 vs. HCV; 13609 ± 2548; p<0.05).
Acknowledgments
The authors are indebted to all the subjects who participated in this study. We also thank Drs. CK Wang, Jennifer Cuthbert, Dwain Thiele and Vinodh Pillai, Ms. Ann Varghese and Ms. Griselda Soto for their help with patient recruitment and Sterling Ortega, Andrew Benagh, Chris Ayers, Dr. Deepani Tennakoon and Dr. Mihail Firan for experimental support and helpful discussions.
Footnotes
These studies were supported, in part, by USPHS NIH grant R01AI047603 (to NJK).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neumann-Haefelin C, Blum HE, Chisari FV, Thimme R. T cell response in hepatitis C virus infection. J Clin Virol. 2005;32:75–85. doi: 10.1016/j.jcv.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Development Conference Statement. Management of hepatitis C: 2002--June 10–12, 2002. Hepatology. 2002;36:S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 3.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–14. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison JG, Alter HJ, Chisari FV. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–76. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 7.Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 9.Pillai V, Lee WM, Thiele DL, Karandikar NJ. Clinical responders to antiviral therapy of chronic HCV infection show elevated antiviral CD4+ and CD8+ T-cell responses. J Viral Hepat. 2006 doi: 10.1111/j.1365-2893.2006.00804.x. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–55. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 11.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, Klenerman P. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–54. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 13.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 14.Ulsenheimer A, Gerlach JT, Gruener NH, Jung MC, Schirren CA, Schraut W, Zachoval R, Pape GR, Diepolder HM. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–98. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 15.Tsubouchi E, Akbar SM, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004;39:754–62. doi: 10.1007/s00535-003-1385-3. [DOI] [PubMed] [Google Scholar]
- 16.Tsubouchi E, Akbar SM, Murakami H, Horiike N, Onji M. Isolation and functional analysis of circulating dendritic cells from hepatitis C virus (HCV) RNA-positive and HCV RNA-negative patients with chronic hepatitis C: role of antiviral therapy. Clin Exp Immunol. 2004;137:417–23. doi: 10.1111/j.1365-2249.2004.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Decreased interferon-alpha production and impaired T helper 1 polarization by dendritic cells from patients with chronic hepatitis C. Clin Exp Immunol. 2004;137:559–65. doi: 10.1111/j.1365-2249.2004.02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccioli D, Tavarini S, Nuti S, Colombatto P, Brunetto M, Bonino F, Ciccorossi P, Zorat F, Pozzato G, Comar C, Abrignani S, Wack A. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–7. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 20.Larsson M, Babcock E, Grakoui A, Shoukry N, Lauer G, Rice C, Walker C, Bhardwaj N. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J Virol. 2004;78:6151–61. doi: 10.1128/JVI.78.12.6151-6161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. Aids. 2005;19:261–71. [PubMed] [Google Scholar]
- 22.Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–26. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 23.Kanto T, Inoue M, Miyazaki M, Itose I, Miyatake H, Sakakibara M, Yakushijin T, Kaimori A, Oki C, Hiramatsu N, Kasahara A, Hayashi N. Impaired function of dendritic cells circulating in patients infected with hepatitis C virus who have persistently normal alanine aminotransferase levels. Intervirology. 2006;49:58–63. doi: 10.1159/000087264. [DOI] [PubMed] [Google Scholar]
- 24.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 25.Stebbing J, Patterson S, Portsmouth S, Thomas C, Glassman R, Wildfire A, Gotch F, Bower M, Nelson M, Gazzard B. Studies on the allostimulatory function of dendritic cells from HCV-HIV-1 co-infected patients. Cell Res. 2004;14:251–6. doi: 10.1038/sj.cr.7290226. [DOI] [PubMed] [Google Scholar]
- 26.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–47. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, Lovett-Racke AE, Frohman EM, Stastny P, Douek DC, Koup RA, Racke MK. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–649. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goutagny N, Fatmi A, De Ledinghen V, Penin F, Couzigou P, Inchauspe G, Bain C. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951–8. doi: 10.1086/375350. [DOI] [PubMed] [Google Scholar]
- 29.Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 30.Sarobe P, Lasarte JJ, Casares N, Lopez-Diaz de Cerio A, Baixeras E, Labarga P, Garcia N, Borras-Cuesta F, Prieto J. Abnormal priming of CD4(+) T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062–70. doi: 10.1128/JVI.76.10.5062-5070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 32.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, Klenerman P. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 34.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–44. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 35.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–72. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–48. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 38.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. T Cells with a CD4+CD25+ Regulatory Phenotype Suppress In Vitro Proliferation of Virus-Specific CD8+ T Cells during Chronic Hepatitis C Virus Infection. J Virol. 2005;79:7860–7. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T Cells Suppress In Vitro Proliferation of Virus-Specific CD8+ T Cells during Persistent Hepatitis C Virus Infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulsenheimer A, Gerlach JT, Jung MC, Gruener N, Wachtler M, Backmund M, Santantonio T, Schraut W, Heeg MH, Schirren CA, Zachoval R, Pape GR, Diepolder HM. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41:643–51. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 41.Bare P, Massud I, Parodi C, Belmonte L, Garcia G, Nebel MC, Corti M, Pinto MT, Bianco RP, Bracco MM, Campos R, Ares BR. Continuous release of hepatitis C virus (HCV) by peripheral blood mononuclear cells and B-lymphoblastoid cell-line cultures derived from HCV-infected patients. J Gen Virol. 2005;86:1717–27. doi: 10.1099/vir.0.80882-0. [DOI] [PubMed] [Google Scholar]
- 42.Pachiadakis I, Pollara G, Chain BM, Naoumov NV. Is hepatitis C virus infection of dendritic cells a mechanism facilitating viral persistence? Lancet Infect Dis. 2005;5:296–304. doi: 10.1016/S1473-3099(05)70114-6. [DOI] [PubMed] [Google Scholar]
- 43.Pal S, Sullivan DG, Kim S, Lai KK, Kae J, Cotler SJ, Carithers RL, Jr, Wood BL, Perkins JD, Gretch DR. Productive replication of hepatitis C virus in perihepatic lymph nodes in vivo: implications of HCV lymphotropism. Gastroenterology. 2006;130:1107–16. doi: 10.1053/j.gastro.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 44.Blackard JT, Smeaton L, Hiasa Y, Horiike N, Onji M, Jamieson DJ, Rodriguez I, Mayer KH, Chung RT. Detection of hepatitis C virus (HCV) in serum and peripheral-blood mononuclear cells from HCV-monoinfected and HIV/HCV-coinfected persons. J Infect Dis. 2005;192:258–65. doi: 10.1086/430949. [DOI] [PubMed] [Google Scholar]
- 45.Castillo I, Rodriguez-Inigo E, Bartolome J, de Lucas S, Ortiz-Movilla N, Lopez-Alcorocho JM, Pardo M, Carreno V. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut. 2005;54:682–5. doi: 10.1136/gut.2004.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L, Fevery J, Hiem Yap S. A novel strand-specific RT-PCR for detection of hepatitis C virus negative-strand RNA (replicative intermediate): evidence of absence or very low level of HCV replication in peripheral blood mononuclear cells. J Virol Methods. 2002;100:97–105. doi: 10.1016/s0166-0934(01)00399-8. [DOI] [PubMed] [Google Scholar]
- 47.Laskus T, Radkowski M, Wang LF, Cianciara J, Vargas H, Rakela J. Hepatitis C virus negative strand RNA is not detected in peripheral blood mononuclear cells and viral sequences are identical to those in serum: a case against extrahepatic replication. J Gen Virol. 1997;78( Pt 11):2747–50. doi: 10.1099/0022-1317-78-11-2747. [DOI] [PubMed] [Google Scholar]
- 48.Feng ZH, Wang QC, Nie QH, Jia ZS, Zhou YX. DC-SIGN: binding receptor for HCV? World J Gastroenterol. 2004;10:925–9. doi: 10.3748/wjg.v10.i7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarobe P, Lasarte JJ, Zabaleta A, Arribillaga L, Arina A, Melero I, Borras-Cuesta F, Prieto J. Hepatitis C virus structural proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J Virol. 2003;77:10862–71. doi: 10.1128/JVI.77.20.10862-10871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 51.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–34. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashi N, Tatsuya Kanto, Tetsuo Takehara. Immunopathogenesis of type C hepatitis: dendritic cells in HCV infection. J Gastroenterol and Hepatology. 2004;19:84–87. [Google Scholar]
- 53.Lucas M, Vargas-Cuero AL, Lauer GM, Barnes E, Willberg CB, Semmo N, Walker BD, Phillips R, Klenerman P. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J Immunol. 2004;172:1744–53. doi: 10.4049/jimmunol.172.3.1744. [DOI] [PubMed] [Google Scholar]
- 54.Leroy V, Bourliere M, Durand M, Abergel A, Tran A, Baud M, Botta-Fridlund D, Gerolami A, Ouzan D, Halfon P, Zarski JP. The antibody response to hepatitis B virus vaccination is negatively influenced by the hepatitis C virus viral load in patients with chronic hepatitis C: a case-control study. Eur J Gastroenterol Hepatol. 2002;14:485–9. doi: 10.1097/00042737-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Navarro JF, Teruel JL, Mateos M, Ortuno J. Hepatitis C virus infection decreases the effective antibody response to hepatitis B vaccine in hemodialysis patients. Clin Nephrol. 1994;41:113–6. [PubMed] [Google Scholar]
- 56.Machida K, Cheng KT, Pavio N, Sung VM, Lai MM. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–89. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D’Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G, Abrignani S. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci U S A. 2005;102:18544–9. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866–74. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batts KP, Ludwig J. Chronic Hepatitis - An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]


