Abstract
The mechanism by which transmyocardial revascularization (TMR) offers clinical benefit is controversial. We hypothesized that TMR ameliorates ischemia by reversing paradoxical catecholamine-induced vasoconstriction. Chronic ischemic cardiomyopathy was created in 11 dogs by placing ameroid constrictors on the proximal coronary arteries and their major branches. Six weeks later, 35 channels were created percutaneously in the left circumflex artery (LCx) region with the left anterior descending artery (LAD) region serving as control. At rest, wall thickening (WT) and myocardial blood flow (MBF) did not change in the treated region, while they deteriorated in the control bed. Contractile and MBF reserve increased in the treated region but deteriorated in the control region. There was diminished 123I-metaiodobenzylguanidine uptake and significant reduction in noradrenergic nerves in the treated region compared to control region, with corresponding reduction in tissue tyrosine hydroxylase activity. We conclude that the absence of catecholamine-induced reduction in MBF reserve and contractile reserve in the TMR treated region with associated evidence of neuronal injury indicates that the relief of exercise-induced ischemia after TMR is most likely due to reversal of paradoxical catecholamine-induced vasoconstriction. These findings may have implications in selecting patients who would benefit from TMR.
Keywords: cardiomyopathy, ischemia, lasers, revascularization
Introduction
Several hypotheses have been put forth to explain angina relief1–9, improved exercise tolerance, and better quality of life8,10–13 after trans-myocardial laser revascularization (TMR). These range from increased myocardial blood flow (MBF) from the left ventricular (LV) cavity via the laser induced-channels14–16 and/or angiogenesis17–21 to pain relief from cardiac denervation22,23.
Unlike normal coronary arteries that exhibit vasodilation during catecholamine stimulation24, injured or diseased coronary arteries may exhibit paradoxical vasoconstriction25–28 leading to reduction in MBF and ischemia during stressful situations. We hypothesized that cardiac denervation caused by TMR would lead to attenuation of paradoxical vasoconstriction, thus ameliorating ischemia, which would explain the angina relief and improved exercise tolerance seen in patients undergoing this procedure. We tested our hypothesis in a canine model of chronic ischemic cardiomyopathy previously developed by us 29,30.
Methods
Animal Preparation
The study protocol was approved by the Animal Research Committee. Eleven mongrel dogs were pretreated with aspirin (75 mg daily) for 3 days, which was continued until euthanasia. They were given Gentamicin (80 mg) and Cefazolin (1 g) immediately prior to surgery and Cefazolin was continued through post-operative day 3. Catheters (6F) were inserted into the right femoral artery for pressure measurements and into the left atrium for injection of microspheres. Up to 4 ameroid constrictors (1.5–3.5 mm) were placed around the dissected left anterior descending coronary artery (LAD) and the left circumflex coronary artery (LCx) and their major branches to create multivessel ischemia. Because the first septal perforator artery in the dog usually comes off the left main artery, it was always spared. As a result, the basal interventricular septum (IVS) never becomes ischemic29,30.
At the completion of procedure, all dogs were treated with buprenorphine (0.15 μg SQ and 0.15 μg IV) and fentanyl transdermal patch (75 mcg·hr−1) for pain control. They were then revived and transferred to the vivarium, where they were examined twice daily and treated appropriately with additional antibiotics or diuretic if infection or heart failure developed. 2D echocardiography (2DE) was performed weekly to assess LV function. All subsequent studies were performed under anesthesia.
Measurement of Regional LV Function and Myocardial Blood Flow
2DE was performed from the right thorax with the dog lying on its left side. Three short-axis views (base, papillary muscle, and apex) were acquired during each study. Care was taken to acquire the same views every time in an individual dog. Wall thickening (WT) was measured offline using a previously defined custom program31. Regional MBF was measured using radiolabeled micro-spheres as previously described32. Normalized MBF was calculated by dividing the averaged MBF of the region of interest by the averaged MBF of the basal IVS (normal segment).
Assessment of Neuron Density with 123I-metaiodobenzylguanidine Imaging
Labeleing of M-iodine-benzyl-guanidine (MIBG), an analog of guanethidine, that is taken up in the presynaptic vesicles similar to norepinephrine was performed with 123I using solid-phase ammonium sulfate exchange.33 Ex vivo 123I-MIBG imaging (159 kev) of myocardial slices corresponding to the 2DE images was performed by placing them directly on the scan-head of a gamma camera. Regions-of-interest were drawn over the LAD and LCx regions at the 3 levels and counts·pixel−1·min−1 were computed and normalized to those at the basal IVS level.
Histopathology and Immunohistochemistry
Samples were stained with hematoxylin and eosin for basic architecture and van Gieson’s picric acid for fibrosis and collagen. Samples were also immunostained with a monoclonal antibody against tyrosine hydroxylase for noradrenergic nerve terminals (BIOMOL)34 and a monoclonal antibody against PECAM-1 for measuring blood vessel density (Santa Cruz). Both tyrosine hydroxylase and PECAM-1 expression were determined by microscopic observation of the diaminobenzidine reaction product (Dako Corp) on the analyzed sections. The regions occupied by fibrosis/collagen (red stain), nerves (dark brown stain), and blood vessels (dark brown stain) were assessed using the following scores based on staining intensity: 0=none, 1=minimal, 2=mild, 3=moderate, and 4=intense. The entire tissue sample (approximately 0.5 cm·0.5 cm) was evaluated for the above changes.
Experimental Protocol
Resting hemodynamic and 2DE data were acquired at baseline and at the time of maximal LV dysfunction (pre-TMR). The pre-TMR evaluation also included MBF and regional WT evaluations at rest and peak dobutamine dose (30–40 μg·kg−1·min−1) to assess MBF and contractile reserves. For TMR approximately 35 channels were created percutaneously in the LCx region under fluoroscopy guidance using the holmium-YAG laser catheter (Eclipse Surgical Technologies, Inc) via a 9F sheath in the right femoral artery. Laser energy was delivered at 5 J per pulse and at a rate of 5 Hz from the 1 mm diameter optical fiber. Maximum penetration (5 mm) was achieved with 3 consecutive pulses and contact was confirmed by occurrence of premature ventricular contractions. After the procedure, the femoral artery was ligated and the incision was closed. The LAD region served as control in these animals.
Hemodynamic and 2DE data were acquired at rest and peak dobutamine stress at 4, 12, and 24 weeks after TMR. At 24 weeks MBF was also assessed at rest and peak dobutamine stress in order to measure MBF reserve. At this time 5 mCi of 123I-MIBG was injected intravenously, and 30 min later the dog was euthanized, the heart was excised, and confirmation of arterial occlusion was achieved by direct examination of the ameroid constrictors. The LV was divided into 0.5 cm short-axis slices of equal thickness. The slices corresponding to the 2DE images were stained for gross infarction.35 These slices were then processed for radioactivity measurements (MBF and 123I-MIBG). The remainder of the tissue was processed for histopathology (approximately 0.5 cm·0.5 cm samples in duplicate).
Statistical Methods
Comparisons between stages were performed using repeated measures ANOVA. The paired Student’s t test was used to determine the difference between 2 stages. A p-value of <0.05 (two-sided) was considered statistically significant for all comparisons.
Results
Maximum LV dysfunction occurred at 8.6±3.6 weeks after placement of the ameroid constrictors, at which time they underwent TMR. All 11 dogs had follow-up studies at 4.7±1.4 and 12±0 weeks; 10 also had a follow-up study at 22.4±2.3 weeks. The 11th animal died unexpectedly after the 12-week follow-up study, but had histopathology performed.
Table 1 shows hemodynamic data at rest and during dobutamine stress. There was no significant change in heart rate, mean aortic pressure, or double product at rest until 12 weeks post-TMR when they increased, probably indicating an increased catecholamine state caused by heart failure. Dobutamine infusion at 30–40 μg·Kg−1·min−1 resulted in a significant increase in heart rate and double product when compared to rest at all time points.
Table 1.
Hemodynamics at Rest and during Dobutamine Stressψ
| Variable | Baseline (n = 11) | Pre-Laser Therapy (n = 11) | 4 weeks post Laser Therapy (n = 11) | 12 weeks post Laser Therapy (n = 11) | 24 weeks post Laser Therapy (n =10 ) |
|---|---|---|---|---|---|
| Rest | |||||
| Heart Rate (beats ·min−1) | 81 ± 27 | 87 ± 24 | 93 ± 20 | 93 ± 20 | 125 ± 26*†‡ |
| Mean Aortic Pressure (mmHg) | 99 ± 19 | 104 ± 17 | 102 ± 16 | 118 ± 17* | 121 ± 25* |
| Double Product (mmHg · beats · min−1) · 100−1 | 115 ± 48 | 118 ± 46 | 127 ± 34 | 119 ± 54 | 143 ± 57 |
| Dobutamine (30–40 μg · Kg−1 · min−1) | |||||
| Heart Rate (beats · min−1) | — | 166 ± 29§ | 139 ± 48*§ | 149 ± 32*§ | 163 ± 23§ |
| Mean Aortic Pressure (mmHg) | — | 116 ± 28§ | 106 ± 12 | 110 ± 33 | 117 ± 15 |
| Double Product (mmHg · beats · min−1) · 100−1 | — | 279 ± 77§ | 280 ± 74§ | 249 ± 103§ | 252 ± 89§ |
Data are presented as mean ± 1SD.
p < 0.05 vs. pre laser therapy
p < 0.05 vs. 4 weeks post laser therapy
p < 0.05 vs. 12 weeks post laser therapy
p < 0.05 vs. rest
WT and MBF at Rest and during Dobutamine Stress
There was no difference in WT between control and treated regions at baseline prior to ameroid constrictor placement (36.5 ± 2.1% vs. 35.9 ± 1.9%, p=0.36). At the point of maximum LV dysfunction pre-TMR, resting WT was worse in the treated compared to the control bed (Figure 1). After TMR, WT did not change further in the treated bed but continued to deteriorate in the control bed. Whereas, dobutamine resulted in improvement of WT in both beds, the improvement was more marked in the treated region (Figure 2). By 24 weeks, contractile reserve in the treated region was 3.5 fold higher than that of the control region.
Figure 1.
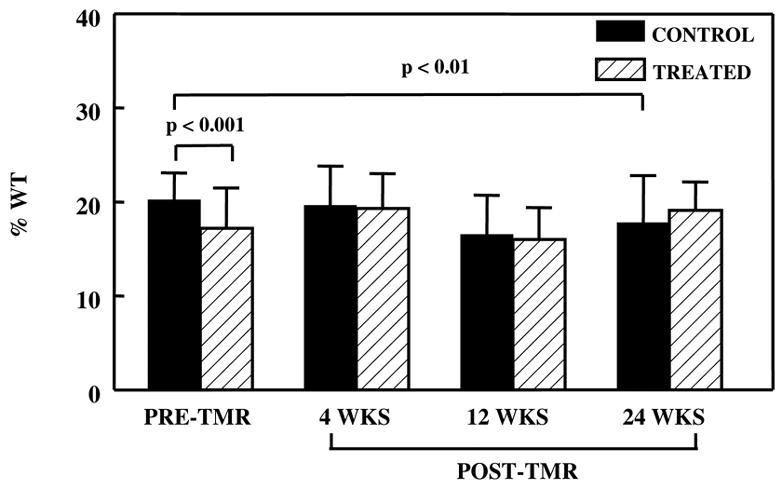
Resting WT in the control and treated regions before and after TMR. In the control region, there was a reduction in WT at 24 weeks post-TMR when compared to pre-TMR. In the treated region, there was no appreciable change.
Figure 2.
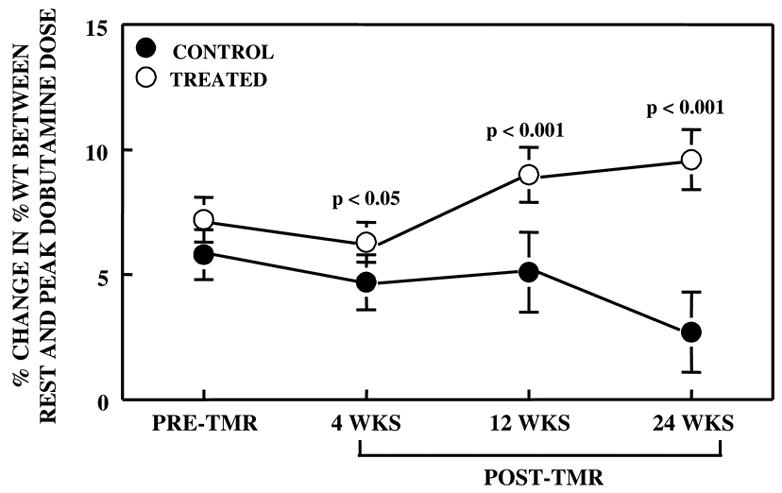
Difference in the change in WT between rest and peak dobutamine dose in both control and treated regions prior to and after TMR. After TMR, myocardial contractile reserve progressively increased in the treated region and progressively decreased in the control region.
Mean resting MBF remained normal in both beds prior to surgery and artery occlusion (baseline) and was unchanged at maximal LV dysfunction (pre-TMR), indicating that the myocardial regions exhibited chronic stunning rather than hibernation. In comparison, MBF at peak dobutamine stress decreased in both the control and treated regions compared to rest (0.66 ± 0.03 versus 0.90 ± 0.67, p<0.05, in the control region; 0.64 ± 0.25 versus 1.06 ± 0.25, p<0.05, in the treated region) indicating the presence of paradoxical vasoconstriction. Resting MBF was not changed 24 weeks post-TMR (Figure 3A); however, dobutamine resulted in a significant increase in MBF in the treated bed (reversal of paradoxical vasoconstriction), but not in the control bed (Figure 3B).
Figure 3.
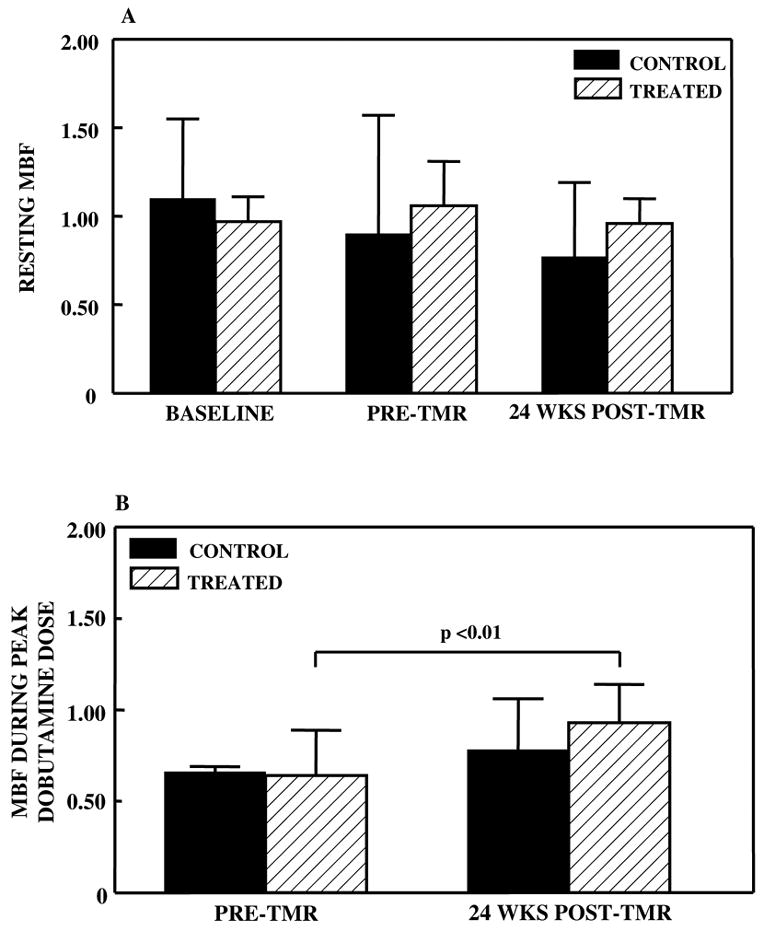
Comparison of normalized transmural MBF before and 24 weeks after TMR in both the control and treated regions at rest (A) and during peak dobutamine dose (B). There was no change in myocardial perfusion at rest, but during dobutamine, there was increased MBF in the treated region.
Neuronal Integrity
Figure 4 is a representative ex-vivo image at the papillary muscle short-axis level after injection with 123I-MIBG. There is less MIBG uptake in the treated LCx than in the control LAD region. Figure 5 illustrates 123I-MIBG counts in the basal IVS as well as papillary muscle and apical levels. There was a significant reduction in 123I-MIBG uptake at the apical levels when compared with the basal IVS. 123I-MIBG counts were also lower in the treated when compared to the control regions at both the papillary muscle and apical levels.
Figure 4.
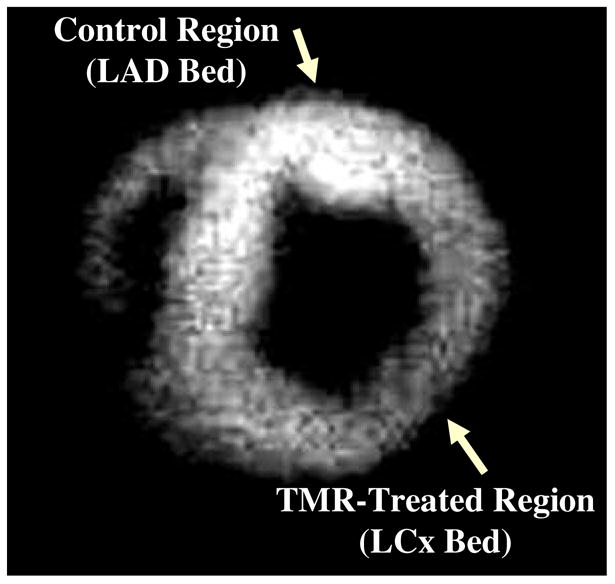
A representative ex-vivo radionuclide image at the papillary muscle short-axis level after injection with 123I-MIBG, which illustrates that there is less uptake in the TMR-treated (LCx) region than in the untreated control (LAD) region.
Figure 5.
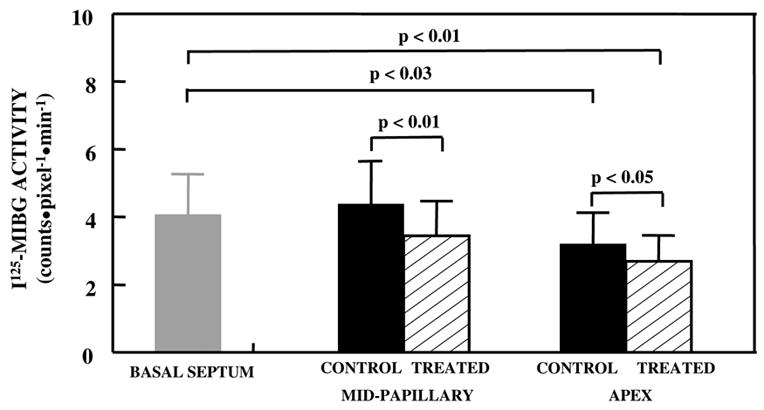
Quantitative 123I-MIBG uptake in the basal IVS, papillary muscle, and apical levels. At the apex, there were significantly reduced counts in the control and TMR-treated regions compared to the basal IVS. At both the papillary muscle and apical levels, the treated region had significantly lower uptake when compared to the control region.
Effects of TMR on Histological Changes
Neither ameroid constriction nor TMR resulted in gross infarction as determined by TTC staining. TMR channels were not seen grossly or microscopically in the 1 dog examined at 12 weeks and the other 10 dogs examined at 24 weeks after the procedure. There was significantly more fibrosis in the TMR-treated compared to the control regions. No fibrosis was seen in the basal IVS (Table 2 and Figure 6A).
Table 2.
Histological results*
| Basal IVS | Mid-PM Control Region | Mid-PM Treated Region | Apex Control Region | Apex Treated Region | |
|---|---|---|---|---|---|
| Picric Acid (Fibrosis) | 0.25 ± 0.16 | 0.25 ± 0.19 | 1.68 ± 0.60* | 01.26 ± 0.61 | 1.51 ± 0.52* |
| Tyrosine Hydroxylase (Noradrenergic Nerve Terminals) | 2.00 ± 0.43 | 0.94 ± 0.38* | 0.63 ± 0.31* | 0.30 ± 0.12* | 0.10 ± 0.06* |
| PECAM-1 (capillaries) | ----- | 3.50 ± 0.50 | 1.87 ± 0.68 | 1.17 ± 0.44 | 0.67 ± 0.33 |
Data are presented as mean ± 1 SEM.
PM=papillary muscle
p < 0.05 vs. basal IVS
Figure 6.
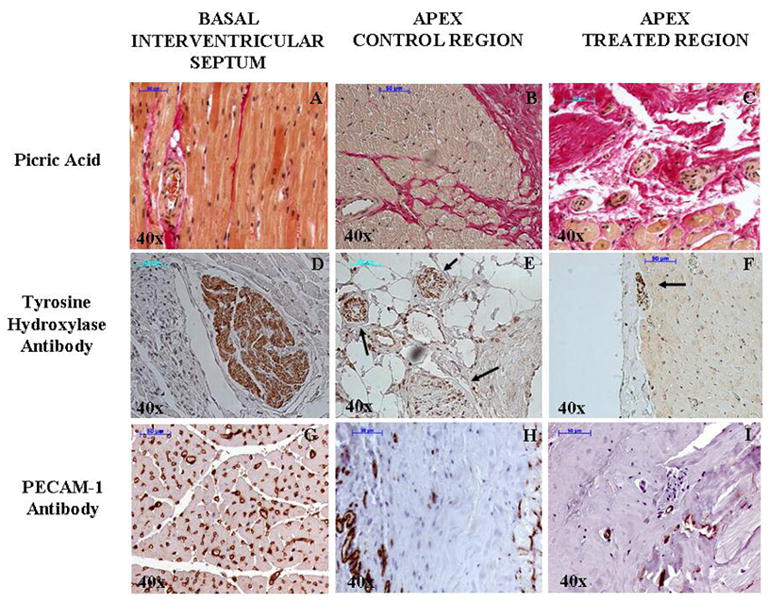
Representative picric acid (A, B, and C), tyrosine hydroxylase antibody (D, E, and F), and PECAM-1 antibody (G, H, and I) staining of myocardial tissue at the basal inter-ventricular spetum and apical levels (control and treated) at 40x magnification. There is significant fibrosis (red) with corresponding reduction of intact nor-adrenergic nerves (dark brown) and blood vessels density (brown) at the apex compared to the basal IVS. The arrows on panels E and F demonstrate nerve bundles with reduced staining.
The untreated basal IVS contained more intact noradrenergic nerve terminals than the 2 lower levels (Figure 6D). The treated regions at both lower levels also exhibited fewer nerve terminals when compared to the control regions at the same levels (Figure 6, E and F). MIBG uptake in the treated regions was also significantly lower than the control region. Capillaries were intact in the basal IVS and were less in the lower 2 levels mostly due to fibrosis. Even within the fibrotic areas, however, less capillaries were seen in the treated compared to the control regions (Figure 6 G, H, and I).
Discussion
The new finding in our study is that paradoxical catecholamine-induced vasoconstriction is reversed after TMR, which may explain the angina relief and increased exercise tolerance reported in patients. We also found evidence of cardiac denervation after TMR, which is very likely to be the proximate cause of the paradoxical catecholamine-vasoconstriction reversal. Furthermore, we confirmed the lack of laser-induced channels or angiogenesis late after TMR, supporting the notion that these are likely not responsible for the clinical benefit seen after TMR.
Our results showing cardiac denervation are consistent with previous reports. One study showed decreased responsiveness of the intrinsic cardiac nervous system at 4 weeks after TMR36. Another clinical study reported diminished uptake of 123I-MIBG after TMR with corresponding reduction in angina during a mean follow-up period of 4.3 months37. Other experimental studies demonstrated minimal denervation after laser therapy38,39, but they were conducted in the acute setting (immediately post and 3 days post-TMR) as opposed to ours, where follow-up period was for 6 months, which is the longest reported time in the literature.
When nerves are transected, degeneration occurs via 2 principal mechanisms: Wallerian degeneration40 and ‘dying back’ degeneration41. In the latter case, degeneration also progresses retrogradely towards the cell body. Thus distally affected nerves also compromise more proximal nerves. Although we did not examine the arterial system for nerve endings, this mechanism seems highly plausible.
Cardiac degeneration also results in upregulation of beta receptors42, which is responsible for the super-sensitivity of the heart to catecholamines43. This super-sensitivity has been documented in cardiac mechanics44 but not in terms of coronary physiology. Denervation seems to have no effect on autoregulation45 or chemoreceptor stimulation in non-disease coronary vessels46. Furthermore, collateral blood flow is not affected by chronic denervation even in myocardial ischemia46,47. Since increase in MBF reserve is not possible in the absence of augmentation of flow through either collaterals or the native coronary arteries, denervation-induced modulation of beta-receptor activity is unlikely to explain our observations.
Other proposed mechanisms
One of the mechanisms proposed early on in the evolution of TMR was that the laser-induced channels were directly responsible for enhancing MBF14–16. It was proposed that these channels connected to pre-existing myocardial vessels and increased nutrient flow. There are several drawbacks to this proposal. The myocardium perfuses mostly in diastole because of the pressure gradient between the aorta and the right atrium. The pressure gradient between the LV cavity and the right atrium is minimal in most of diastole and so appreciable flow to the myocardium from the LV cavity is unlikely to occur. In systole the increased myocardial impedance prevents any filling of myocardial vessels whether from the LV cavity or the native vessels. More recently it has been shown that these channels close a few weeks after the procedure and are replaced by fibrosis48. Our results confirm these findings.
Another proposed mechanism is angiogenesis17–21. It has been postulated that inflammation and/or injury induced by laser stimulates new vessel formation. We not only did not see any new vessels but compared to the control region, we saw a paucity of capillaries in the treated bed, which has significant fibrosis induced by laser.
Critique of our Methods
The dogs were not randomized to treatment of the LAD versus the LCx beds. The LAD region served as the control region in all dogs because the pre-determined curvature of the laser catheter did not allow us to access the LAD bed reproducibly. Rather than performing an incomplete series of treatments in the LAD region, we elected to treat only the LCx region, where we were confident that we could accurately deliver laser therapy to a large area of myocardium.
Prior to TMR, the control myocardium (LAD region) had higher baseline wall thickening compared to the soon-to-be treated myocardium (LCX region) probably because of additional blood flow from collaterals that formed from the first septal perforator to the LAD.
The observed increase in MBF in the control group at 24 weeks post-TMR is probably due to flow from collateral vessels that formed in this region. It is known that the vasomotion of collateral blood vessels differs from the native coronary vasculature. Since the extent of adrenergic innervation is substantially reduced in collateral vessels and the architecture of their smooth muscle is altered during development, they probably do not vasoconstrict in the presence of catecholamine like native diseased vessels
Clinical Implications
We believe that our findings are likely to apply to humans because in the setting of chronic CAD, both coronary circulations are similar in that there is an abundance of collateral vessels. Furthermore, in humans, myocardial denervation has already been demonstrated after laser treatment using PET imaging with [11C]hydroxyephedrine, an inactive norepinephrine analog that is a highly specific tracer of presynaptic nerve terminals22 and MIBG imaging37. Even though the mechanism of paradoxical vasoconstriction has not been fully elucidated, it is plausible that the sympathetic nervous system is involved. When the network of nerves is disrupted, it follows that function would also be lost. Our postulate will need to be confirmed in the clinical setting.
Not all patients benefit from TMR. Our results indicate that relief of TMR is related to reversal of paradoxical vasoconstriction that may or may not be present in all diseased coronary arterial segments. Maneuvers that could elicit paradoxical vasoconstriction (such as hand-grip exercise25 or cold-pressor test28) which in turn could induce regional ischemia (evidenced by inducible regional dysfunction on 2DE) could help select patients that would most likely benefit from TMR. Controlled clinical studies are required to prove this point.
Conclusion
The absence of catecholamine-induced reduction in MBF reserve and contractile reserve in the TMR treated region with associated evidence of neuronal injury indicates that the relief of exercise-induced ischemia after TMR is most likely due to reversal of paradoxical catecholamine-induced vasoconstriction. These findings may have implications in selecting patients who would benefit from TMR.
Acknowledgments
The authors would like to thank Mrs. Linda G. Bailes for her expert technical assistance. They would also like to express their gratitude to Mr. John P. Huberty for providing MIBG and Ms. Melissa Bevard, Mr. Zachary Gregg, and Mr. Ajay Kadakkal, for their assistance with histology and immuno-histochemistry.
Footnotes
Supported in part by grants from the National Institutes of Health (R01-HL66034 and K-08-HL074290-01), Bethesda, Maryland. The radiolabeled microspheres were provided by Dupont Pharmaceuticals, North Billerica, Massachusetts, and the ultrasound equipment was supplied by Philips, Andover, Massachusetts. Dr. Leong-Poi was the recipient of a Fellowship Training Grant from the Canadian Institute of Health Research and the Heart and Stroke Foundation of Canada, Ottawa, Canada.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frazier OH, Cooley DA, Kadipasaoglu KA, Pehlivanoglu S, Lindemeier M, Barasch E, et al. Myocardial revascularization with laser. Preliminary findings. Circulation. 1995;92(Suppl II):II58–65. doi: 10.1161/01.cir.92.9.58. [DOI] [PubMed] [Google Scholar]
- 2.Cooley DA, Frazier OH, Kadipasaoglu KA, Lindenmeier M, Pehlivanoglu S, Kolff JW, et al. Transmyocardial laser revascularization: clinical experience with twelve-month follow-up. J Thorac Cardiovasc Surg. 1996;111:791–799. doi: 10.1016/s0022-5223(96)70339-2. [DOI] [PubMed] [Google Scholar]
- 3.Horvath KA, Mannting F, Cummings N, Shernan SK, Cohn LH. Transmyocardial laser revascularization: operative techniques and clinical results at two years. J Thorac Cardiovasc Surg. 1996;111:1047–1053. doi: 10.1016/s0022-5223(96)70381-1. [DOI] [PubMed] [Google Scholar]
- 4.Horvath KA, Cohn LH, Cooley DA, Crew JR, Frazier OH, Griffith BP, et al. Transmyocardial laser revascularization: results of a multicenter trial with transmyocardial laser revascularization used as sole therapy for end-stage coronary artery disease. J Thorac Cardiovasc Surg. 1997;113:645–654. doi: 10.1016/S0022-5223(97)70221-6. [DOI] [PubMed] [Google Scholar]
- 5.Kornowski R, Baim DS, Moses J, Hong MK, Laham RJ, Fuch S, et al. Short- and intermediate-term clinical outcomes from direct myocardial laser revascularization guided by biosense left ventricular electromechanical mapping. Circulation. 2000;102:1120–1125. doi: 10.1161/01.cir.102.10.1120. [DOI] [PubMed] [Google Scholar]
- 6.Milano A, Pratali S, Tartarini G, Mariotti R, De Carlo M, Paterni G, et al. Early results of transmyocardial revascularization with a holmium laser. Ann Thorac Surg. 1998;65:700–704. doi: 10.1016/s0003-4975(97)01380-5. [DOI] [PubMed] [Google Scholar]
- 7.Donovan CL, Landolfo KP, Lowe JE, Clements F, Coleman RB, Ryan T. Improvement in inducible ischemia during dobutamine stress echocardiography after transmyocardial laser revascularization in patients with refractory angina. J Am Coll Cardiol. 1997;30:607–612. doi: 10.1016/s0735-1097(97)00219-2. [DOI] [PubMed] [Google Scholar]
- 8.Oesterle S, Sanborn TA, Ali N, Resar J, Ramee SR, Heuser R, et al. Percutaneous myocardial laser revascularization for severe angina: the PACIFIC randomised trial. Lancet. 2000;356:1705–1710. doi: 10.1016/s0140-6736(00)03203-7. [DOI] [PubMed] [Google Scholar]
- 9.Lauer B, Junghans U, Stahl F, Kluge R, Oesterle SN, Schuler G. Catheter-based percutaneous myocardial laser revascularization in patients with end-stage coronary artery disease. J Am Coll Cardiol. 1999;34:1663–1670. doi: 10.1016/s0735-1097(99)00419-2. [DOI] [PubMed] [Google Scholar]
- 10.Allen KB, Dowling RD, Fudge TL, Schoettle GP, Selinger SL, Gangahar DM, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med. 1999;341:1029–1036. doi: 10.1056/NEJM199909303411403. [DOI] [PubMed] [Google Scholar]
- 11.Burkhoff D, Schmidt S, Schulman SP, Myers J, Resar J, Becker LC, et al. Transmyocardial laser revascularization compared with continued medical therapy for treatment of refractory angina pectoris: a prospective randomized trial. ATLANTIC investigators: Angina Treatments-Lasers and Normal Therapies in Comparison. Lancet. 1999;354:885–890. doi: 10.1016/s0140-6736(99)08113-1. [DOI] [PubMed] [Google Scholar]
- 12.Frazier OH, March RJ, Horvath KA. Transmyocardial revascularization with carbon dioxide laser in patients with end-stage coronary artery disease. N Engl J Med. 1999;341:1021–1028. doi: 10.1056/NEJM199909303411402. [DOI] [PubMed] [Google Scholar]
- 13.Schofield PM, Sharples LD, Caine N, Burns S, Tait S, Wistow T, et al. Transmyocardial laser revascularization in patients with refractory angina: a randomized controlled trial. Lancet. 1999;353:519–524. doi: 10.1016/s0140-6736(98)11478-2. [DOI] [PubMed] [Google Scholar]
- 14.Deckelbaum LI. Cardiovascular applications of laser technology. Lasers Surg Med. 1994;15:315–341. doi: 10.1002/lsm.1900150402. [DOI] [PubMed] [Google Scholar]
- 15.Mirrhoseini M, Muckerheide M, Cayton MM. Transventricular revascularization by laser. Lasers Surg Med. 1982;2:187–198. doi: 10.1002/lsm.1900020209. [DOI] [PubMed] [Google Scholar]
- 16.Cooley DA, Frazier OH, Kadipasaoglu KA, Pehlivanoglu S, Shannon RL, Angelini P. Transmyocardial laser revascularization: anatomic evidence of long-term channel patency. Tex Heart Inst J. 1994;21:220–224. [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes GC, Lowe JE, Kypson AP, et al. Neovascularization after transmyocardial laser revascularization in a model of chronic ischemia. Ann Thorac Surg. 1998;66:2029–2036. doi: 10.1016/s0003-4975(98)01095-9. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto N, Takushi K, Anguo G, DeRosa C, Smith CR, Burkhoff D. Angiogenesis is enhanced in ischemic canine myocardium by transmyocardial laser revascularization. J Am Coll Cardiol. 1998;31:1426–1433. doi: 10.1016/s0735-1097(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 19.Chu VF, Giaid A, Kuang J, McGinn AN, Li CM, Pelletier MP, et al. Angiogenesis in transmyocardial revascularization: comparison of laser versus mechanical punctures. Ann Thorac Surg. 1999;68:301–308. doi: 10.1016/s0003-4975(99)00680-3. [DOI] [PubMed] [Google Scholar]
- 20.Hughes GC, Kypson AP, Annex BH, Yin B, St Louis JD, Biswas SS, et al. Induction of angiogenesis after TMR: a comparison of holmium:YAG, CO2, and excimer lasers. Ann Thorac Surg. 2000;70:504–509. doi: 10.1016/s0003-4975(00)01569-1. [DOI] [PubMed] [Google Scholar]
- 21.Domkowski PW, Biswas SS, Steenbergen C, Lowe JE. Histological evidence of angiogenesis 9 months after transmyocardial revascularization. Circulation. 2001;103:469–471. doi: 10.1161/01.cir.103.3.469. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shiekh T, Allen KB, Straka SP, Heimansohn DA, Fain RL, Hutchins GD, et al. Cardiac sympathetic denervation after transmyocardial laser revascularization. Circulation. 1999;100:135–140. doi: 10.1161/01.cir.100.2.135. [DOI] [PubMed] [Google Scholar]
- 23.Minisi AJ, Topaz O, Quinn MS, Mohnaty LB. Cardiac nociceptive reflexes after trans-myocardial laser revascularization: implications for the neural hypothesis of angina relief. J Thorac Cardiovasc Surg. 2001;122:712–719. doi: 10.1067/mtc.2001.116946. [DOI] [PubMed] [Google Scholar]
- 24.Barbato E, Bartunel J, Wyffels E, Wijns W, Heyndrickx GR, De Bruyne B. Effects of intravenous dobutamine on coronary vasomotion in humans. J Am Coll Cardiol. 2003;42:1596–1601. doi: 10.1016/j.jacc.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation. 1984;70:18–24. doi: 10.1161/01.cir.70.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Gage JE, Hess OM, Murakami T, Ritter M, Grimm J, Krayenbuehl HP. Vasoconstriction of stenotic coronary arteries during dynamic exercise in patients with classic angina pectoris: reversibility by nitroglycerin. Circulation. 1986;73:865–876. doi: 10.1161/01.cir.73.5.865. [DOI] [PubMed] [Google Scholar]
- 27.Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, et al. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest. 1989;83:1946–52. doi: 10.1172/JCI114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Firoozan S, Wei K, Linka A, Skyba D, Goodman NC, Kaul S. A canine model of chronic ischemic cardiomyopathy: characterization of regional flow-function relations. Am J Physiol. 1999;276:H446–H455. doi: 10.1152/ajpheart.1999.276.2.H446. [DOI] [PubMed] [Google Scholar]
- 30.Pelberg RA, Spotnitz WD, Bin J-P, Le E, Goodman NC, Kaul S. Mechanism of Myocardial Dysfunction inthe Presence of Chronic Coronary Stenosis and Normal Resting Myocardial Blood Flow: Clinical Implications. J Am Soc Echocardiogr. 2001;14:1047–1056. doi: 10.1067/mje.2001.113232. [DOI] [PubMed] [Google Scholar]
- 31.Sklenar J, Jayaweera AR, Kaul S. A computer-aided approach for the quantification of regional left ventricular function using two-dimensional echocardiography. J Am Soc Echocardiogr. 1992;5:33–40. doi: 10.1016/s0894-7317(14)80100-4. [DOI] [PubMed] [Google Scholar]
- 32.Heymann MA, Payne BD, Hoffman JI, Rudolph AM. Blood flow measurements with radionuclide labeled particles. Prog Cardiovasc Dis. 1997;20:52–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- 33.Dae MW, O’Connell JW, Botvinick EH, Ahearn T, Yee E, Huberty JP, et al. Circulation. 1989;79:634–644. doi: 10.1161/01.cir.79.3.634. [DOI] [PubMed] [Google Scholar]
- 34.Himura Y, Felten S, Kashiki M, Lewandowski TJ, Delehanty JM, Liang C-S. Cardiac nor-adrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–1309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- 35.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, et al. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 36.Arora RC, Hirsch GM, Hirsch MD, Armour JA. Transmyocardial laser revascularization remodels the intrinsic cardiac nervous system in a chronic setting. Circulation. 2001;104(suppl I):I115–I120. doi: 10.1161/hc37t1.094901. [DOI] [PubMed] [Google Scholar]
- 37.Beek JF, van der Sloot JAP, Huikeshoven M, Verberne HJ, van Eck-Smit BL, van der Meulen J, et al. Cardiac denervation after clinical transmyocardial laser revascularization: Short-term and long-term iodine 123-labeled meta-iodobenzylguanine scintigraphic evidence. J Thorac Cardiovasc Surg. 2004;127:517–524. doi: 10.1016/s0022-5223(03)00973-5. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch GM, Thompson GW, Arora RC, Hirsch KJ, Sullican JA, Armour JA. Transmyocardial laser revascularization does not denervate the canine heart. Ann Thorac Surg. 1999;68:460–469. doi: 10.1016/s0003-4975(99)00558-5. [DOI] [PubMed] [Google Scholar]
- 39.Johnson LL, Thambar S, Donahay T, Dae M, Williams DO. Effect endomyocardial laser channels on regional innervation shown with 125I-MIBG and autoradiography. J Nucl Med. 2002;43:551–555. [PubMed] [Google Scholar]
- 40.Wallerian Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 41.Luo L, O’leary DDM. Axon retraction and degeneration in health and disease. Ann Rev Neurosci. 2005;28:127 –156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 42.Valette H, Deleuze P, Syrota A, Delforge J, Crouzel C, Fuseau C, et al. Canine myocardial beta-adrenergic, muscarinic receptor densities after denervation: a PET study. J Nucl Med. 1995;36:140–146. [PubMed] [Google Scholar]
- 43.Vatner DE, Lavelle M, Amano J, Finizola A, Homcy CJ, Vatner SF. Mechanisms of super-sensitivity to sympathomimetic amines in the chronically denervated heart of the conscious dog. Circ Res. 1985;57:55 –64. doi: 10.1161/01.res.57.1.55. [DOI] [PubMed] [Google Scholar]
- 44.Vergroesen I, Merkus D, van Teeffelen JWGE, Dankelman J, Spaan JAS, van Wezel HB, et al. Chronic cardiac denervation affects the speed of coronary vascular regulation. Cardiovasc Res. 1999;44:615 –622. doi: 10.1016/s0008-6363(99)00257-6. [DOI] [PubMed] [Google Scholar]
- 45.Nagata M, pichet R, Lavallee M. Coronary dilation with carotid chemoreceptor stimulation in cardiac-denervated dogs. Am J Physiol. 1988;255:H1331 –H1335. doi: 10.1152/ajpheart.1988.255.6.H1330. [DOI] [PubMed] [Google Scholar]
- 46.Shen YT, Knight DR, Vatner SF, Randall WC, Thomas JX. Responses to coronary artery occlusion in the conscious dog with selective cardiac denervation. Am J Physiol. 1988;255:H525 –H533. doi: 10.1152/ajpheart.1988.255.3.H525. [DOI] [PubMed] [Google Scholar]
- 47.Shen YT, Knight DR, Thomas JX, Vatner SF. Effects of selective cardiac denervation on collateral blood flow after coronary artery occlusion in conscious dogs. Bas Res Cardiol. 1990;85(suppl 1):229–239. doi: 10.1007/978-3-662-11038-6_19. [DOI] [PubMed] [Google Scholar]
- 48.Kohmoto T, Fisher PE, Gu A, Zhu SM, DeRosa CM, Smith CR, et al. Physiology, histology, and 2-week morphology of acute transmyocardial channels made with a CO2 laser. Ann Thorac Surg. 1997;63:1275–1283. doi: 10.1016/s0003-4975(97)00102-1. [DOI] [PubMed] [Google Scholar]


