Abstract
OBJECTIVES
The prevalence and characteristics of fetal alcohol syndrome (FAS) and partial fetal alcohol syndrome (PFAS) were determined in a third primary school cohort in a community in South Africa (S.A.).
METHODS
An active case ascertainment, two-tier screening methodology, and the revised Institute of Medicine diagnostic criteria were employed among 818 first grade pupils. Characteristics of children with FAS and PFAS are contrasted with a randomly-selected control group. Data were collected and analyzed for children in the study regarding: 1.) physical growth and development, including dysmorphology, 2.) intelligence and behavioral characteristics, and 3.) their mother’s social, behavioral, and physical characteristics.
RESULTS
The rate of FAS and PFAS in this area continues as the highest reported in any overall community and is much higher than rates elsewhere. In this cohort it is 68.0 to 89.2 per 1,000. Severe episodic drinking on weekends among mothers of children with FAS and PFAS accounts for 96% of all alcohol consumed. Various measures of maternal drinking are significantly correlated with negative outcomes of children in the areas of non-verbal intelligence (-0.26), verbal intelligence (-0.28), problem behavior (0.31), and overall dysmorphology score (0.59). Significantly more FAS and PFAS exists among children of rural residents (OR = 3.79).
CONCLUSIONS
A high rate of FAS and PFAS was again documented in this community, and it has increased. Given population similarities, we suspect that other communities in the Western Cape Province of South Africa also have high rates. Programs for prevention are needed.
Keywords: fetal alcohol syndrome, epidemiology, South Africa, alcohol abuse
1.0 Introduction
Alcohol is a teratogen that has raised concerns about birth outcomes for years (Sullivan, 1899;Lemoine, et al., 1968; Abel, 1998; Armstrong, 2003), but the diagnosis of fetal alcohol syndrome (FAS) was not formalized and published until 1973 (Jones and Smith, 1973). Further delineation of the diagnosis has occurred since then, and work on its nature and applications continues (Sokol and Clarren, 1989; Aase, 1994; Aase, et al., 1995; Stratton, et al., 1996; Astley and Clarren, 2000; Hoyme, et al., 2005).
FAS is a pattern of anomalies and developmental deficits found in children exposed to large amounts of alcohol in the prenatal period. Children with FAS have a characteristic pattern of facial and body dysmorphology, delayed physical growth and development, and specific mental and behavioral deficits (Stratton, et al., 1996). For a diagnosis of FAS, all three categories of problems must be present (Stratton, et al., 1996), and the diagnosis should be made only after excluding other genetic and teratogenic anomalies (Hoyme, et al., 2005). Even though a FAS diagnosis can be made without confirmation of maternal drinking (Stratton, et al., 1996), a detailed maternal history is best to confirm the quantity, frequency, and timing of gestational drinking (May, 1995). In this study we provided diagnoses of two Institute of Medicine (IOM) prescribed fetal alcohol outcomes, FAS and partial fetal alcohol syndrome (PFAS) (Stratton, et al., 1996). In addition to these two levels of fetal alcohol outcomes, the IOM report defined alcohol-related neurodevelopmental deficits (ARND) where the child has neurodevelopmental delays and/or behavioral problems with minimal dysmorphology characteristic of FAS or alcohol-related birth defects (ARBD) with some dysmorphology but minimal behavioral or neurodevelopmental problems consistent with FAS (Stratton, et al., 1996). The entire continuum of effects, from mild to severe, is called fetal alcohol spectrum disorder (FASD).
1.1. South Africa and FAS Rates Elsewhere Placed in Perspective
In a first active case ascertainment study in this South African community, the rate of FAS among first grade children was 40.5 to 46.4 per 1,000 (May, et al., 2000). In a second, similar study it was even higher two years later at 65.2 to 74.2 per 1,000. Both of these rates of prevalence are the highest ever reported in the world.
FAS prevalence from clinic-based studies is estimated for the United Sates (U.S.) as 0.33 to 2.2 (Abel, and Sokol, 1991; Stratton, et al., 1996; May and Gossage, 2001), and an average estimate for the developed world is 0.97 per 1,000 (Abel and Sokol, 1987; Abel, 1998). A clinic-based rate of FAS for African Americans of low socioeconomic status (SES) has been reported as 2.3 per 1000 (Abel, 1998, 1995). In a few high risk American Indian reservation communities in the U.S., the prevalence of FAS has been researched via studies utilizing active case ascertainment methods, and averages of 8 per 1,000 have been found. The short-term prevalence in these populations sometimes exceeds 20 per 1,000 (Abel, 1995; May, 1991; May, et al., 1983; 2002).
Active case ascertainment for FAS epidemiological research, where outreach and aggressive case finding are practiced, was endorsed by a study committee of the U.S. Institute of Medicine as the most accurate approach to ascertaining prevalence; but such studies are logistically challenging, expensive, and time consuming (Stratton, et al., 1996). Nevertheless, estimations of FAS prevalence and most of the delineation of the characteristics of all levels of FASD in the U.S. come from various other sources: birth records, disability registries, clinic-based studies, and a very few population-based initiatives (Chavez, et al., 1988; Stratton, et al., 1996; May, 1996: Burd, 1996; Egeland, et al., 1995; 1998; Kvigne, et al., 2004). All but one of the four active case ascertainment studies of FASD in the U.S., where outreach in major geographical areas focuses on aggressive case finding, were carried out among American Indians (Quaid, et al., 1993; Duimstra, et al.,1993; May, et al.,1983;Clarren, et al., 2001). Passive, record-based systems and clinic-based methods which investigate FAS among clients presenting for medical services (e.g., prenatal clinics) are most commonly used in studies of other U.S. and European populations (Abel and Sokol, 1991, 1987; Abel, 1995; May, 1996; Chavez, et al., 1988; Egeland, et al., 1995, 1998). Therefore, because of wide variation in methodologies, comparison of FAS prevalence and the characteristics of children with FASD is difficult to impossible.
1.2 The Background of the Study Region
Fruit orchards, grape-growing, wine production, and agriculture dominate large areas of Western Cape Province (WCP) of South Africa (SA). Wine production and unique social, and economic conditions over the past 300 years have influenced the modal drinking patterns. Wine was historically distributed daily by farmers to workers as partial payment for labor among an impoverished labor force. This practice was called the “Dop” system. Dop was outlawed over the past 40 years by multiple statutes, and there is widespread public sentiment against it. In our last study of this area, less than 2% of mothers of FAS children reported receiving Dop. But residual patterns of frequent, heavy, episodic alcohol consumption (now commercially purchased) by some individuals and groups remain a legacy of the Dop system. In recent years there have been changes in the nature of the drinking behavior of the worker population, as increased contemporary availability of inexpensive commercial beer, wine, and liquor, primarily in “take-away” (carry-out) sources and shebeens (illegal bars), has maintained or exacerbated heavy, episodic drinking behavior (London, et al., 1995; Parry, 1998; London, 2000; Crome and Glass, 2000; Mager, 2004). Episodic (binge) drinking is a major form of recreation among sub-segments of the WCP population, which contributes to many public health problems (King et al., 2004).
The population of the WCP is four million people composed of: 57% Cape Coloured (mixed race), 18% Black, 25% White, and 1% other. The Cape Town metropolitan area is the principal urban area of the WCP, but 40% of the population lives outside of it in small towns and rural areas. The study community is similar in social and economic character to many others in the Winelands of WCP, with a 1996 population of 45,255 (28.1% rural) (RSA Census, 1997). Most of the inhabitants of this area are Coloured (people of mixed ancestry from intermarriage of black African populations, European-origin whites, and some Asians).
This article summarizes a third, active case ascertainment FAS research initiative in a first grade cohort in the WCP. Although FAS had been diagnosed in S.A. before (Palmer, 1985), a first population-based study in this community was prompted by a bi-national (U.S. and S.A.) commission initiated by the Vice Presidents of the two countries (NIAAA, 1996; 1998). A follow-up study was undertaken in 1999, and both waves of research revealed the highest rates of FAS ever reported. While answering a number of questions about the nature and causes of FAS in this community in general, other questions were raised or were reframed by the findings (May, et al., 2000; Viljoen, et al., 2005). The study reported upon here was designed to further explore and summarize the epidemiology, maternal risk, child characteristics, trends, and etiology of FAS and PFAS in this community and on FASD in other human populations as well.
2.0 Methods
2.1 Diagnostic Criteria
Diagnostic components of the revised U.S. Institute of Medicine (IOM) categories (Hoyme, et al., 2005) were used to assess FAS and PFAS in the children. For the diagnosis of FAS a child must have: 1.) evidence of a characteristic pattern of minor facial anomalies including at least 2 or more of the key facial features of FAS (palpebral fissures ≤10th centile, thin vermilion border, or smooth philtrum), 2.) evidence of prenatal and/or postnatal growth retardation (height (hgt.) or weight (wgt.) ≤10th centile), 3.) evidence of deficient brain growth (structural brain anomalies or occiptofrontal head circumference (OFC) ≤10th centile), and if possible, 4.) confirmation of maternal alcohol consumption directly from the mother or a knowledgeable collateral source. For a diagnosis of Partial FAS (PFAS), a child must have: 1.) evidence of a characteristic pattern of facial anomalies including 2 or more of the three mentioned above, 2.) one or more other characteristics, such as prenatal or postnatal growth retardation (≤10th centile) in height or weight), 3.) small OFC (≤ 10th centile), and/or evidence of a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplainable by genetic composition, family background, or environment alone, and if possible, 4.) confirmation of maternal alcohol consumption directly from the mother or a collateral source. There was no attempt in this study to diagnose any of the other, less dysmorphic (usually less severe) forms of the IOM-defined fetal alcohol spectrum, alcohol-related birth defects (ARBD) or alcohol-related neurodevelopmental disorder (ARND), as the study sampling methods were keyed by growth and structural deficits not obviously present in children with ARND or ARBD. Every child with a FASD diagnosis met each of the IOM criteria for FAS or PFAS, and criteria for alcohol exposure during pregnancy were met in 94.7% of all FASD cases from direct maternal interview and 100% from collateral sources.
2.2 Sampling - The Two-Tier Screening System and Children Participating in Each Stage
In the first study in this community (May, et al., 2000), dysmorphology, growth, and developmental data for 406 unselected first grade children were collected initially to provide child size and growth norms for this particular population relative to National Center for Health Statistics (NCHS) growth charts and knowledge of the local clinical traits. The unique racial admixture of the WCP necessitated this first step. The growth and clinical data from the first study were utilized primarily to: calibrate the growth norms, standardize the observations of the clinicians, set the cut off criteria for Tier II screening of the study, and ensure capture of all FAS children residing within the study population.
Twelve of the 13 elementary schools of the community were accessed in this wave. The 12 participating primary schools constitute all of the public schools in the area. Six schools are in rural areas and primarily educated the farm laborers’ children. Six are in the town. Since urban schools are larger, almost 70% of the children attended the urban schools. All but one of the schools were attended by over 95% Coloured and Black students, the one exception was an urban school with a highly mixed racial/ethnic composition of about 50% White. The school that declined participation was a private, all white school with approximately 60 upper socioeconomic status first grade students. Therefore, over 85% of the children in the study were Coloured; the remainder are Blacks or Whites, similar to the regional population. Most of the study children underwent gestation locally due to relatively low mobility in the population.
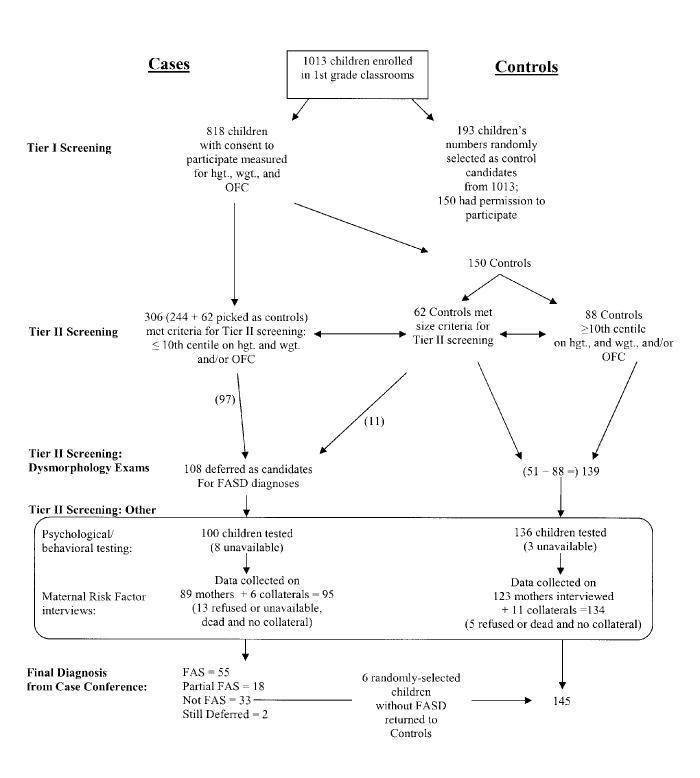
In this study, 818 (80.8%) of 1013 children on the rolls in first grade classrooms had parental consent to participate. Each received Tier I screening where hgt., wgt., and OFC were measured. If at or below the 10th centile on OFC and/or on both hgt. and wgt., a child was referred for a complete physical examination (Tier II). Three hundred and six (37.4%) children met these criteria (see Figure 1). A general surveillance of institutions for children with retardation or other developmental disabilities (who were not in normal schools) did not reveal any children with FAS or PFAS from the study area who were of appropriate age for first grade.
Figure 1.
Sampling Methodology and Flow of Referrals for Children in Wave 3 of the Western Cape, South Africa Study
2. 3 Sampling-Tier II
Every child receiving a complete (Tier II) dysmorphology screen was methodically examined by two pediatric dysmorphologists working simultaneously, but independently, each along with one scribe (data recorder). Each of these experienced physicians measured OFC, palpebral fissure length (PFL), philtral length (PL), inner and outer canthal distance (ICD and OCD), and examined other indicators such as joint abnormalities, heart function, and palmar creases while the scribe recorded the findings on a standardized form (Hoyme, et al., 2006). All examiners were blinded from prior knowledge of child and mother. Once finished with one physician, a second team repeated the examination. Inter rater reliability for independent measurements was assessed in the two previous waves of our SA studies. Using the square root of the Pearsonian Correlation, results were: 0.86 for ICD, 0.92 for IPD, 0.91 for PFL, and 0.82 for PL (Viljoen, et al., 2005), indicating substantial reliability.
2.4 Sampling - The Complete Diagnostic Sequence Continues
After the dysmorphology exams, a child was assigned a preliminary diagnosis of not-FAS, deferred, or FAS. Only those with the classic FAS phenotype and measurements were assigned a preliminary diagnosis of FAS after major symptoms of other known birth defects had been ruled out. Children with a deferred diagnosis had the appearance, growth deficits, and some anomalies of FAS, but developmental tests and maternal interviews were especially necessary for final diagnosis. Children with a preliminary FAS or deferred diagnosis (n = 108) were advanced to intelligence, behavioral, and developmental testing, and their mothers were interviewed for prenatal risks.
2.5 Sampling - Control Group
The control group of children provides a normative comparison group typical of the general population of the community. Initially, 193 children were randomly selected as possible controls from lists of all 1013 first grade students enrolled in these schools. Some of the children selected did not have parental consent to participate (n = 43). Also, a number of the selected controls (n = 62) were small and therefore met criteria for Tier II dysmorphology exams. But after the dysmorphology exams were completed, all but 11 of the 62 were returned to control status, as their morphology was not consistent with FAS or PFAS. The 11 controls who remained in the FASD subject group at this point then went through the rest of Tier II screening (developmental testing and maternal interview). Six of these children were found not to have FAS or PFAS and, therefore, returned to control status. At the end of this study, five of the initially-selected controls converted to a diagnosis of FAS or PFAS, leaving 150 possible control children. Some children moved from the area or were otherwise unavailable during testing, leaving a final control sample of 145 children. This represents 75.1% of those picked randomly from the total pupil population. When differences between the selected controls and the total sample of students were examined for eight key variables via t-tests, (sex, age, hgt., wgt., BMI for age, BMI, BMI centile, and OFC), there were no significant differences. This indicates that the controls are generally representative of the total sample with consent to participate. Regarding those 19% who did not participate in the overall study, we have no way of knowing if they are fundamentally different from those who consented. Given the relatively homogeneous nature of the population, however, we do not think so. Furthermore, a participation rate of 81% in an active consent in-school study of any kind is an exceptionally high rate.
Identical Tier II exams and testing were performed on all subjects and controls, and clinicians were blinded throughout the process as to reason for entry into the study.
2.6 Developmental Tests - Psychological and Intellectual Functioning of the Children
Developmental tests administered to all children in Tier II of the study included: Tests of the Reception of Grammar (TROG), Raven Colored Progressive Matrices, and the Personal Behavior Checklist (PBCL-36). The TROG (Bishop, et al., 1989) tests linguistic understanding through language comprehension and mastery of a fundamental intellectual developmental trait. The TROG provides age-specific assessment of verbal I.Q. Non verbal I.Q. is measured by the Raven Test of Coloured Progressive Matrices (Alderton and Larson, 1990) which assesses thinking in the visual modality as a measure of inductive reasoning with broad cross-cultural applicability. The PBCL-36 is a short scale that measures the behavioral characteristics of FAS, regardless of age, race, sex, or I.Q. (Streissguth, et al., 1998). It consists of 36 items in a checklist pertaining to several areas of functioning: academic performance, social skills and interactions, bodily functions, communication and speech, personal manner, emotions, and motor skills and activities. The PBCL is completed by the mother or another guardian. These three instruments provided measures of verbal and non-verbal I.Q., cognitive skills, and behavioral problems.
2.7 Maternal Risk Data - Measuring Alcohol/Substance Abuse, Demographic, and Social Variables
The mothers of control children became maternal controls. Structured interviews contained items covering: reproduction; alcohol, tobacco, and drug use, before and during the index pregnancy; socio-economic status (SES); demographic variables; nutrition; physical status of the mother; and social context.
Protocols utilized drinking questions formulated and arranged in a timeline follow-back methodology (Sobel, et al., 1988; 2001) designed to elicit accurate reporting of alcohol consumed from both Dop and commercial sources (London, et al., 1998; London, 2000; Parry and Bennetts, 1998). Photographs of standard beer, wine, and spirit containers and tobacco products sold locally were shown to respondents for accurate assessment of quantity, frequency, and variability pf drinking (Kaskutas & Graves, 2000, 2001). A 7-day retrospective drinking log was used as the baseline for all alcohol questions to calibrate quantity and frequency of recent drinking (current alcohol consumption), and for establishing an accurate benchmark for recall of relative amounts of drinking during pregnancy of the index child (see May, et al., 2000; 2005; Viljoen, et al., 2002; 2005). This method was used because direct reporting of prenatal drinking is generally under-reported (Czarnecki, et al., 1990) and retrospective reports of maternal drinking are generally significantly higher than that reported during the prenatal period (Czarnecki, et al., 1990; Alvik, et al., 2006; Jacobson, et al., 1991; 2002; Jacobson and Jacobson, 1994). Women may underreport their alcohol use during pregnancy unless information is gathered through unique questions, methods, and contextual frameworks such as part of a general nutrition screen (King, 1994). Drinking questions, therefore, followed the nutrition questions in our interviews. All maternal interviews were administered in the field by experienced Afrikaans-speaking professionals.
Tobacco use (cigarettes) of the mothers was queried. Since most people in this population purchased loose tobacco and rolled their own cigarettes with newspaper, it was necessary to calculate the amount of tobacco per cigarette and then ask the mothers about how much they purchased each week and smoked per day. We provided standard commercial (12.5 and 25 gram) pouches to several local women and asked them to roll all of the tobacco in each pouch into cigarettes as they would normally. The cigarettes contained an average of one gram of tobacco each. Therefore, in Table 3 each gram of tobacco equals one cigarette.
TABLE 3.
Substance Use by Mothers and Fathers of the Children with FASD and Randomly-selected Controls: Wave III.
| Variable | Mothers Of Control With FASD1 (n = 61) | Mothers Of Children Children (n = 133) | P, OR | Statistical Test | (95% CI)d |
|---|---|---|---|---|---|
| Current drinker in last year (%) | 61.0 | 26.9 | p<0.001a, OR = 4.25 | x2 = 20.11 | (2.10 - 8.66) |
| Current use of alcohol, whole sample, | |||||
| last week - mean (SD) | 3.2 (7.1) | 1.1 (4.2) | 0.013b | F = 6.23 | |
| weekend - mean (SD) | 3.1 (6.8) | 0.7 (3.1) | 0.001b | F = 10.07 | |
| weekend (% of all drinks) | 96.8 | 72.7 | NS (0.587)c | x2 = 0.30 | |
| Current use of alcohol, drinkers only | |||||
| last week - mean (SD) | 7.5 (9.4) | 8.9 (8.5) | NS (0.636)b | F = 0.23 | |
| weekend - mean (SD) | 7.2 (8.9) | 6.5 (6.8) | NS (0.806)b | F = 0.06 | |
| weekend (% of all drinks) | 96.0 | 73.0 | NS (0.238)c | x2 = 1.39 | |
| Drinking before index pregnancy (%) | 92.3 | 25.6 | p<0.001a, OR = 34.94 | x2 = 78.00 | (12.0 - 1109.3) |
| Drank during index pregnancy (%) | 95.5 | 24.2 | P<0.001a, OR = 65.63 | x2 = 89.40 | (17.9 - 285.1) |
| Drank during index trimester (%) | |||||
| 1st | 93.9 | 23.3 | p<0.001a, OR = 51.00 | x2 = 88.40 | (15.9 - 181.9) |
| 2nd | 83.6 | 16.5 | p<0.001a, OR = 25.69 | x2 = 84.17 | (10.8 - 62.3) |
| 3rd | 79.1 | 15.8 | p<0.001a, OR = 20.19 | x2 = 76.62 | (8.9 - 46.6) |
| Current smoker, whole sample (%) | 75.8 | 35.6 | p<0.001a, OR = 5.65 | x2 = 28.39 | (2.85 - 11.7) |
| Quantity of tobacco used per week (mean grams) | |||||
| Whole sample | 28.9 (37.5) | 10.8(19.9) | p<0.001a | F =18.77 | |
| Smokers only | 38.5 (38.8) | 33.4 (21.9) | NS (0.456)a | F = 0.56 | |
| Smoked during index pregnancy (%) | 83.8 | 35.3 | p<0.001a, OR = 9.48 | x2 = 42.36 | (4.3 - 21.4) |
| Fathers | |||||
| Drank during index pregnancy (%) | 81.1 | 47.5 | p<0.001a, OR = 4.74 | x2 = 11.72 | (1.7 - 13.6) |
| Drinkers - drinks per 30 days, mean (SD) | 166.2 (211.5) | 147.7 (300.4) | NS(0.776) | F = 0.08 | |
| Fathers with drinking problems in past (%) | 13.4 | 4.8 | p<0.035a, OR = 3.05 | x2 = 4.44 | (0.9 - 10.3) |
| Fathers with current drinking problems (%) | 11.1 | 0.8 | p<0.001a, OR = 15.00 | x2 = 10.54 | (1.8 - 337.9) |
Mortality and mobility reduced the number of available mothers of FAS children by 10. See Methods Section text for details.
NS = not statistically significant.
X2 test.
t-test.
Difference of proportions test
95% confidence intervals calculated via the Cornfield technique.
In this part of South Africa, average hand-rolled cigarettes equal one gram of tobacco.
Drank “less,” the “same amount,” or “more” than use at time of interview.
Smoked “less,” “the same amount,” or “more” than use at time of interview
Study participants were also asked about other drug use. Less than 1% reported any drug use other than alcohol at interview or during pregnancy. The most common other drug reported was Daka, the S.A. name for marijuana. Prescription drugs are rarely available or used in this population.
2.8 The Maternal Samples - Mothers of FASD Children and Controls
Since mothers of children with FAS often lead chaotic lives, death or mobility obviated some interviews (May, et al., 1983; Streissguth, et al., 1985). Specifically, 57 of the 73 mothers of the children with FASD were located; all agreed to be interviewed, which rarely occurs in similar studies elsewhere (May, et al., 2006). Six (8.2%) FASD case mothers were deceased (one each from tuberculosis, heart failure, liver failure, alcohol abuse, suicide, and motor vehicle crash). The remaining 10 were nomadic or moved, and could not be reached. Most of the children with FASD born to the mothers not interviewed were in foster or adoptive placement, often precipitated by alcohol abuse and the disrupted lives of the mothers. For these 16 not interviewed directly, some data regarding alcohol consumption during the index pregnancy were obtained via collaterals (usually relatives) on all but 2. Therefore we had some information, either direct or indirect, on the drinking status during the index pregnancy of all but 2 (97.3%) of the mothers of children with FASD. Detailed profiles and discussion of a variety of maternal risk factors for FAS in this setting are reported elsewhere (Viljoen, et al., 2002; May, et al., 2005). Only three (5.3%) of the 57 mothers of children with FASD interviewed denied drinking during the index pregnancy. To ensure accuracy, two dysmorphologists revisited these three children to rule out other anomalies and to confirm the diagnosis. With 3 interviewees denying drinking and 2 missing data, 5 (6.8%) children were diagnosed as FAS or PFAS without confirmed alcohol exposure from any source.
Because the maternal control group consisted of the mothers of the randomly-selected children, it was also random. It was intended to provide a comparison group that is representative of the general population of the area, and consisted of the 145 mothers of the randomly-selected control children located alive and agreeing to interviews. Eighty-five percent of the mothers of the control children were interviewed directly. The data on these mothers constitute the basis of most comparisons in this manuscript. Of the 22 randomly-selected mothers not interviewed, only 6 (4.1%) refused an interview, which is a low refusal rate in such a sensitive topic of study. One control mother (0.7%) was known to be deceased, 3 (2.1%) had moved away, and 12 were not located for other reasons (e.g. 5.3% of control children were in foster or adoptive placement). Collateral data on drinking were obtained for only some of the missing control mothers (n=11).
2.9 Case Conferences for Final Diagnoses
Final diagnoses were made only after case conferences were held for each child (Hoyme, et al., 2005; Viljoen, et al., 2005). Results from the three independently-collected data sets (dysmorphology exams, developmental testing, and maternal interviews) were presented at a structured case conference held in the Western Cape. Participants at the case conference were the researchers who had collected the data. At least one person represented each domain studied. Prior to the review of data in the case conference, the independent investigators of each domain remained blinded to the data collected on each child by investigators in the other domains.
2.10 Data Analysis
Data were entered and analyzed using the EPI Info software of the U.S. Centers for Disease Control and Prevention (Dean, et al., 1996). Categorical variables comparing cases to controls were analyzed by chi-square and also Fisher’s Exact tests where appropriate. Odds Ratios (OR) were calculated for all 2 x 2 chi-square tests, and are not possible for other chi-square configurations. Confidence intervals for Odds Ratios were computed for the 95% level by the Cornfield technique. For continuous variables, t-tests, one way analysis of variance, and difference of proportions tests (Blalock, 1972) were used. When analysis of variance tests were significant, post-hoc analyses were performed with t-tests for all possible individual pairs. In Table 4, Pearson correlation coefficients are used to compare selected variables, three of which were utilized as dummy (0=no, 1=behavior practiced) variables. These items were whether the woman drank at all during pregnancy and whether she reported having 3 or more or 5 or more drinks per occasion during pregnancy. With certain variables, comparisons are made between subsets of cases and controls based on current drinking and smoking and on others based on drinking during the index pregnancy. The timing of the drinking is specified by labels in the Tables and text. Alpha levels for significance were set at .05 with two-tailed comparisons.
TABLE 4.
Pearson Correlation Coefficients for Developmental1 and Physical Dysmorphology vs. Selected Reported Drinking Measures During Pregnancy Among Mothers (n = 191)
| Trait | Reported Drinking During Pregnancy | Drinks Per Month | Drinks Per Day | 3 Drinks Per Occasion | 5 Drinks Per Occassion |
|---|---|---|---|---|---|
| Verbal abilitya | -0.21** | -0.20** | -0.20** | -0.28*** | -0.21** |
| Non-verbal abilityb | -0.17**** | -0.25*** | -0.26*** | -0.25*** | -0.26*** |
| Behaviorc | 0.31*** | 0.24*** | 0.23** | 0.31*** | 0.20** |
| Dysmorphology score | 0.59*** | 0.43*** | 0.48*** | 0.51*** | 0.45*** |
All scores standardized for age of child at time of testing.
Tests of the Reception of Grammar (TROG).
Raven Colored Progressive Matrices.
Personal Behavior Checklist (PBCL36).
p <.01
p <.001
p<.05
3.0 Results
3.1 Physical Growth and Development
In Table 1, column 1, data are presented for all of the children who had consent to participate in the study. Of the 818 children examined, 51.5% were male. The mean age of the first grade children was 7.3 years (87.8 months). Children averaged 119 cm in height, weighed 21.8 kg, and had OFC of 50.9 cm. After the dysmorphology exam, 30 children had a preliminary diagnosis of FAS, and 78 were preliminarily deferred pending further indicators for a more definite diagnosis. These 108, along with the 145 control children, were the subjects of further research (see Figure 1).
TABLE 1.
Demographic and Growth Parameters for all 1st Grade Children, Children with FASD and Randomly-selected Controls: Western Cape Community, South Africa: Wave III.
| Variable | All Sub-A Children (N = 818) | Control Children** (n =145) | Children with Partial FAS (n = 18) | Children with FAS (n = 55) | Statistical Test | P |
|---|---|---|---|---|---|---|
| Sex (%) | ||||||
| Males | 51.5 | 47.6 | 61.1 | 58.2 | ||
| Females | 48.5 | 52.4 | 38.9 | 41.8 | x2 = 2.54 NS | (0.281)a |
| Age (months) | ||||||
| Mean (SD) | 87.8 (6.56) | 87.5 (6.29) | 91.6 (6.63)2 | 93.7(10.28)3 | F = 14.33 | <0.001b |
| Height (cm) | ||||||
| Mean (SD) | 119.0 (5.95) | 119.0 (5.95)* | 114.8 (4.51)2 | 114.3 (4.92)3 | F = 16.59 | <0.001b |
| Weight (kg) | ||||||
| Mean (SD) | 21.8 (3.75) | 21.8 (3.58)* | 19.3 (2.29)2 | 18.3 (2.41)3 | F = 26.18 | <0.001b |
| Average BMI for Age | ||||||
| Mean (SD) | 15.5 (0.21) | 15.5 (0.22) | 15.6 (0.18) | 15.7 (0.39)3 | F = 11.13 | <0.001b |
| Child’s BMI | ||||||
| Mean (SD) | 15.3 (1.72) | 15.3 (1.66) | 14.6 (1.10) | 13.9 (1.16)3,4 | F = 17.67 | <0.001b |
| BMI Percentile | ||||||
| Mean (SD) | 38.6 (26.57) | 39.6 (25.14) | 24.4 (19.65)1 | 14.4 (15.82)3,4 | F = 25.45 | <0.001b |
| Occipital Circumference (OFC) (cm) | ||||||
| Mean (SD) | 50.9 (1.70) | 50.9 (1.35)* | 49.5 (1.25)3 | 48.3 (1.19)3,6 | F = 79.20 | <0.001b |
| Palpebral Fissure Length (cm) | ||||||
| Mean (SD) | - | 2.5 (0.12) | 2.3 (0.09)3 | 2.3 (0.11)3,5 | F = 96.53 | <0.001b |
| Philtrum Length (cm) | ||||||
| Mean (SD) | - | 1.4 (0.22)* | 1.4 (0.24) | 1.4 (0.21) | F = 0.61 | NS (0.542)b |
| Short Intercanthal Distance (%) | - | 15.9 | 0.0 | 30.9 | x2 = 10.44 | 0.005a |
| Short Interpupilary Distance (%) | - | 15.9 | 0.0 | 30.9 | x2 = 10.44 | 0.005a |
| Hypoplastic Midface (%) | - | 19.3 | 50.0 | 45.5 | x2 = 17.87 | <0.001a |
| “Railroad Track” Ears (%) | - | 2.8 | 16.7 | 14.5 | x2 = 11.58 | 0.003a |
| Strabismus (%) | - | 0.7 | 0.0 | 5.5 | x2 = 5.39 | NS (0.067)a |
| Ptosis (%) | - | 2.1 | 5.6 | 18.2 | x2 = 17.25 | <0.001a |
| Epicanthal Folds (%) | - | 48.7 | 55.6 | 61.8 | x2 = 2.41 | NS (0.299)a |
| Flat Nasal Bridge (%) | - | 26.9 | 66.7 | 49.1 | x2 = 16.69 | <0.001a |
| Anteverted Nostrils (%) | - | 11.7 | 44.4 | 20.0 | x2 = 13.08 | 0.001a |
| Smooth Philtrum (%) | - | 60.7 | 83.3 | 87.3 | x2 = 15.06 | 0.001a |
| Narrow Vermillion Border (%) | - | 40.7 | 83.3 | 85.5 | x2 = 38.51 | 0.001a |
| Prognathism (%) | - | 2.1 | 0.0 | 10.9 | x2 = 8.72 | NS (0.013)a |
| Heart Murmur (%) | - | 3.4 | 22.2 | 10.9 | x2 = 10.67 | 0.005a |
| Heart Malformations (%) | - | 0.0 | 5.6 | 1.8 | x2 = 6.09 | 0.048a |
| Hypoplastic Nails (%) | - | 1.4 | 0.0 | 10.9 | x2 = 10.99 | 0.004a |
| Limited Elbow Supination (%) | - | 0.7 | 11.1 | 3.6 | x2 = 8.35 | 0.015a |
| Clinodactyly (%) | - | 43.8 | 61.1 | 50.9 | x2 = 2.79 | NS (0.248)a |
| Camptodactyly (%) | - | 8.3 | 27.8 | 36.4 | x2 = 23.95 | 0.001a |
| Palmar Crease Alteration (%) | - | 26.2 | 38.9 | 43.6 | x2 = 6.07 | 0.048a |
| Hypertrichosis (%) | - | 0.0 | 5.6 | 0.0 | x2 = 11.16 | 0.004a |
| Dysmorphology Score | ||||||
| Mean (SD) | - | 8.1 (4.19) | 17.8(3.92)3 | 18.4 (3.30)3 | F = 160.36 | <0.001b |
| Children in Foster Care or Adoptive Placement (%) | - | 5.3 | 33.3 | 20.4 | x2 = 17.23 | <0.001a |
NS = not statistically significant.
X2 test of data comparing children with FAS, Partial FAS, and controls.
ANOVA of data comparing children with FAS, Partial FAS, and controls.
Measurements at time of Tier I screen; therefore they are directly comparable to all other groups.
t-tests were used to compare the control group (n = 139) to the remainder of the total sample (n = 679) and in none of the comparisons was the control group significantly different from the overall child population. Comparison variables were: sex, age, height, weight, average BMI for age, BMI, BMI percentile, or head circumference.
t-test significantly different from controls; p <.05.
t-test significantly different from controls; p <.01.
t-test significantly different from controls; p <.001.
t-test significantly different: PFAS vs. FAS; p <.05.
t-test significantly different: PFAS vs. FAS; p <.01.
t-test significantly different: PFAS vs. FAS; p <.001.
Fifty-five (55) of the school children received a final diagnosis of FAS, 18 a final diagnosis of PFAS and 2 initially-deferred children were considered “still deferred” due to an inability to locate them for testing (see Figure 1). Five of the randomly selected control children were ultimately diagnosed with FAS (1) or PFAS (4). Each of these 5 also met Tier II screening criteria because of small size of hgt. wgt. and/or OFC. In Table 1 (columns 2 - 4), there is variation in the sex composition of the FAS, PFAS, and control groups, but the difference was not statistically significant. The average age was 7.3 years for controls, 7.6 for PFAS, and 7.8 years for the FAS children. Height, weight, BMI measures, OFC, palpebral fissure length, and most every clinical exam variable was significantly different between FAS, PFAS, and controls, including total dysmorphology scores (18.4 for FAS, 17.8 for PFAS, vs. 8.1 for controls p < .001). Higher dysmorphology scores indicate more features of FAS. Measurements of philtrum length were not significantly different between FAS and PFAS children in the SA population, primarily because a long philtrum is very common in the Coloured and Black population. Also, clinodactyly was found to be very common in the overall population, and therefore was not a good discriminator between the children with FASD and controls in this population. However, other hand anomalies such as camptodactyly and an altered palmar crease were significantly different between the groups. Other traits not statistically different between groups were all clinical observations made without quantitative or structured criteria: strabismus, epicanthal folds, and prognathism. Nevertheless, with each of these variables, the clinical indicators were higher in the FASD children than among controls. Significantly more of the children with FAS (20.4%) and PFAS (33.3%) were in foster or adoptive placement than controls (5.5%).
Post hoc analysis of the significant differences between FAS, PFAS, and control groups in Table 1 indicated that both the PFAS group and especially the FAS group were different from controls on most interval level measures of growth, development, and dysmorphology. The total dysmorphology scores of the PFAS and FAS groups were significantly different at the < .001 level (see footnotes in Table 1). Significant differences between FAS and PFAS groups were found in child’s BMI, BMI centile, head circumference, and palpebral fissure length.
3.2 Other Developmental Indicators
Table 2 presents comparative data for children with FAS, PFAS and controls. The children with FAS scored worse than the children with PFAS or controls on all of the developmental tests in Table 2. Verbal and non-verbal ability were significantly lower for children with an FASD than for controls using one way analysis of variance (p < .001), although the post-hoc, individual t-test comparison between PFAS and controls only approached significance (p=.06). Problem behaviors were also higher than, and statistically significant from, controls for both groups of FASD when measured by ANOVA and individual t-tests. The overall dysmorphology scores, indicating severity of physical deformity and lack of physical development, form a spectrum; but, the two FASD groups are quite close in their high severity of dysmorphology (see Table 2). Children with FAS had the highest average score of 18.4, then children with PFAS (17.8), and then the controls (7.8). There were no statistically significant differences between the FAS and PFAS groups on any of the four developmental traits in Table 2. Therefore, while intelligence scores, behavioral indicators, and dysmorphology were all consistent in exhibiting a spectral pattern, the significant differences were only between the two FASD groups and the controls.
TABLE 2.
Mean Scores on Developmental and Behavioral Indicators* of Children with Fetal Alcohol Syndrome (FAS) and Partial Fetal Alcohol Syndrome (PFAS) Compared to Randomly-selected Controls: Wave III.
| Final Dx FASD |
||||||
|---|---|---|---|---|---|---|
| Child Variables | FAS (SD) (n = 55) | PFAS (SD) (n = 18) | Controls (SD) (n= 133) | Test Score | Df | p |
| Developmental Traits | ||||||
| Verbal IQa | 10.9 (15.72)2 | 14.0 (15.94) | 24.1 (21.46) | F = 9.31 | df = 2/199 | <0.001 |
| Non-verbal IQb | 9.4 (9.1)2 | 10.7 (9.6)1 | 21.1 (18.9) | F = 11.43 | df = 2/199 | <0.001 |
| Behaviorc | 12.6 (8.82)2 | 12.2 (6.16)1 | 6.5 (7.91) | F = 12.96 | df = 2/198 | <0.001 |
| Total Dysmorphology Score** | 18.4 (3.29)2 | 17.8 (3.92)2 | 8.1 (4.19) | F = 160.36 | df = 2/209 | <0.001 |
| Maternal Variables | (n = 50) | (n = 16) | (n = 132) | |||
| Drinking Indicator - overall Reported drinking during pregnancy (%) | 96.0 | 93.8 | 24.2 | X2 = 89.42 | df = 2 | <0.001 |
| Average No. drinks per week (during pregnancy)** | 13.0 (13.62)1 | 4.9 (3.45) | 6.0 (7.71) | F = 4.26 | df = 2/75 | .018 |
| Current drinker** (%) | 65.9 | 53.8 | 60.0 | X2 = 0.68 | df = 2 | NS (0.71) |
| Average No. drinks per week (current)** | 7.8 (10.03) | 6.3 (5.19) | 9.1 (5.67) | F = 0.15 | df = 2/31 | NS (0.86) |
NS = Not statistically significant
All scores standardized for age of child at time of testing.
Of those who reported drinking during pregnancy in a full interview.
t-test significantly different from controls; significance < .05
t-test significantly different from controls, p< .001
Tests of the Reception of Grammar (TROG).
Raven Colored Progressive Matrices.
Personal Behavior Checklist (PBCL36).
Reports of maternal drinking during pregnancy, also in Table 2, further support the spectrum of damage associated with physical development and behavioral problems. The reported drinking during pregnancy was significantly different between the three groups: 96% of the mothers of children with FAS, 93.8% of the PFAS, and 24.2% of controls. The mean number of drinks consumed per week during pregnancy by mothers who reported some drinking was also significantly different. The mothers of children with FAS reported the most at 13, then the controls who drank (6), with the mothers of PFAS lowest at 4.9. There is no significant difference in reported current drinking with 53.8 to 65.9% drinking in each of the three groups. Also, on number of drinks consumed per current week, control mothers who were drinking at the time of the interview reported the greatest number. But the current drinking levels are not significantly different between groups. Thus the spectrum of drinking does not hold across all of these measures with the three maternal groups, as 24% of control mothers who drank during pregnancy and the 60% of controls who are current drinkers report higher levels of drinking than do the mothers of PFAS children. In post hoc analysis only one significant difference was found in individual comparisons between the three groups on the maternal drinking variables in Table 2. The number of drinks during pregnancy reported by mothers of children with FAS and controls was significantly different (p=.02), and the difference between FAS and PFAS on this variable approached significance (p=.07).
3.3 Maternal Drinking and Smoking and the Link the FASD Outcome
In Table 3 data on alcohol use and smoking compare mothers of FASD children and controls. Data for FAS and PFAS were combined for this comparison because the differences in the two FASD maternal groups did not differ significantly on current drinking or drinking during pregnancy responses between FAS and PFAS maternal groups. Other maternal drinking variables (Table 3) indicate that more mothers of children with an FASD were drinking greater quantities of alcohol at the time of interview (61% vs. 26.9%). Furthermore, over 96% of all alcohol currently consumed by mothers of children with FASD was on weekends, while 73% of the controls’ drinking was on weekends. This was not a significant difference. Weekend drinking is a common regional pattern. Ninety-two percent of the mothers of children with FASD reported drinking immediately prior to the index pregnancy compared to 26% for controls (p<.001). Furthermore, 96% of mothers of children with FASD and 24% of the controls reported consuming alcohol during pregnancy (p<.001). Ninety-four percent of the mothers of children with FASD drank during the first trimester, 84% in the second trimester, and 79% drank during the third, which differed significantly the 23% to 16% for controls (p<.001 for all trimesters). The beverage of choice reported by the mothers of children with FASD (not in Table 3) was beer (81.8%), followed by wine (53.3%), and distilled spirits (6.1%). These percentages exceed 100 because many mothers reported more than one favorite beverage.
More mothers of children with FASD used tobacco in the current time period, at the time of interview (75.8% vs. 35.6%, p<.001, OR = 5.65) and during the index pregnancy (83.8% vs. 35.3%, p<.001, OR = 9.48). Similar significant differences existed between groups through each trimester, although the data are not reported in Table 3. In spite of high prevalence of prenatal smoking, by American and European standards, smokers (in both groups) consumed low mean quantities each week: 33 to 39 hand-rolled cigarettes.
Fathers of children with FAS or PFAS and the fathers of controls are reported by female respondents to drink heavily. While 81.1% of case fathers drank during the index pregnancy compared to 47.5% of controls, a significant difference, the mean quantity of drinks consumed by drinking fathers of the two groups were high during the index pregnancies (166 to 148 per month) and did not differ significantly. There was tremendous variability in the drinking levels in both groups as indicated by the large standard deviations. Fathers of children with FASD were more likely than controls to be described as having had a drinking problem, (p=.035, OR = 3.05) and to currently have a drinking problem (p=.001, OR = 15.00).
Presented in Table 4 are zero-order correlations between four drinking measures and specific outcomes; all associations were significant at various levels. Verbal and non-verbal ability are negatively correlated with the mother’s reported drinking during pregnancy: whether she reported drinking at all during the index pregnancy, drinks per month, drinks per day, and reported episodes of 3 or 5 alcoholic drinks per day during gestation. Poor verbal scores are most highly correlated with heavy episodic drinking of 3 or more drinks per day (-0.28) and low non-verbal scores with number drinks per day (-0.26) and five or more drinks per occasion (-.26). The more drinking reported per day during pregnancy, the lower the child’s I.Q., especially verbal. Problem behaviors were significantly correlated with all measures. Heavy drinking mothers, especially those who reported drinking episodes of 3 drinks or more per occasion during pregnancy (0.33) had children with more behavioral problems. The highest correlations in Table 4 were found between total dysmorphology scores and all drinking measures, especially with the dummy variable of whether the mothers reported drinking at all (0.59) and episodes of 3 drinks or more drinks per occasion (0.51).
3.4 Urban/Rural Distribution and Prevalence of FAS
Mothers of children with FASD were much more likely than controls to have resided in rural areas during gestation (See Table 5). Mothers of children with FASD are more likely to be farm laborers than controls (40.6% vs. 19.8%, p = .006). When direct maternal reports are examined, 65.2% reported residing in rural areas during the index pregnancy compared to 33.1% of controls (OR= 3.75). The urban/rural distribution of FAS cases was also tested against the overall residence pattern through indirect standardization (Barclay, 1958). Since 65% (n = 45) of the FAS cases underwent gestation in the rural areas, this is a significant departure from random distribution (p < .001, OR = 4.93), as only 28% of the population of the study site lived in rural areas.
TABLE 5.
Distribution of Gestation of FAS Cases by Rural and Urban Calculated from Interviews and by Indirect Standardization and Overall Prevalence of FAS: Wave III, 2002.
| Variable | Mothers Of Children With FASD1 (n = 69) | Mothers Of Control Children (n = 134) | P, OR (95% CI)c |
|---|---|---|---|
| Mother’s usual occupation Farm laborer (%) | 40.6 | 19.8 | 0.006 |
| Residence at time of index pregnancy (from interview) | |||
| Rural | 65.2 (n = 45) | 33.3 (n = 44) | <0.001a, OR = 3.75 |
| Urban | 34.8 (n = 24) | 66.7 (n = 88) | (1.93 - 7.32) |
| Residence of FAS and PFAS mothers (indirect standardization by frequency) | Rural | Urban | |
| Actual | 45 | 24 | <0.001a, OR = 4.93 |
| Predicted | 19 | 50 | (2.25 - 10.91) |
| Prevalence Rates (FAS & PFAS) | |||
| 1st grade children screened | 89.2 per 1,000 | ||
| 1st grade children per all enrolled in 12 schools | 72.1 per 1,000 | ||
| 1st grade children per all enrolled in all schools | 68.0 per 1,000 | ||
Mortality and mobility reduced the number of available mothers of FAS children by 4. See Methods Section text for details.
NS = not statistically significant.
X2 test.
t-test.
95% confidence internals calculated via the Cornfield technique.
The prevalence of FAS among children screened (n = 818) was 89.2 per 1,000. A conservative calculation of the rate is 72.1 if all 1013 first graders children in public schools are used as the denominator (See Table 5). Although all races in the public school were screened, no white children were found to have FAS or PFAS in this third wave of research. There were few older children in first grade in this study cohort; therefore, there was no need to correct for age. If the approximately 60 children in first grade from the private, all-White school that did not participate are added to the denominator, and it is assumed that none of them have FAS, the most conservative, in-school prevalence is 68.0 per 1,000. Since no out-of-school children were identified with FAS, the range of FAS prevalence for the community was therefore 68.0 to 89.2 per 1,000 children.
4.0 DISCUSSION
In this third wave of research in the Western Cape Province community, the prevalence of FAS and PFAS was found to be extremely high overall, and highest in the impoverished rural areas. Participation in the study continued at a very high rate (81%). Children with FAS and PFAS had significantly higher scores for dysmorphology than controls and performed significantly worse on verbal I.Q., non-verbal I.Q., and the behavioral measures of the PBCL. Ninety-six percent of the mothers of FAS children, 94% of mothers of PFAS children, and 24% of controls reported drinking during pregnancy. With the reported levels of current drinking among those who were drinkers at the time of interview, quantity per week was highest among mothers of FAS (13.0 ± 13.6), lowest among the mothers of PFAS children (4.9 ± 3.5), and intermediate among controls (6.0 ± 7.7). A much higher percentage of the mothers of FASD children than controls are current drinkers (61 vs 27%) and mothers of FASD children report substantially lower drinking levels at time of interview than reported drinking during the index pregnancy. A much higher percentage of the mothers of FASD children report smoking during pregnancy (84%) vs controls (35%), but the quantity of cigarettes smoked per week by the smokers in both groups is low (39 and 33). Fathers of FASD children are 1.7 times more likely to drink during index pregnancies (81 vs. 48%), and are significantly more likely to be reported as problem drinkers in past and present. Measures of drinking during the index pregnancies are significantly associated with low intelligence and frequent behavioral problems in the children. Reported drinking during pregnancy (.59), drinks per day (.48), 3 drinks or more per occasion (.51), and 5 drinks or more per occasion (.45), correlate highly with total dysmorphology in the children. Finally, the rate of FAS and PFAS was found to still be the highest reported in the world at 68.0 to 89.2 per 1,000, slightly higher than a previous wave of research carried out in 1999. No other major community in the world has been found to have such high rates of FASD.
4.1 Limitations of the Study
While this is the most complete and comprehensive population-based study of FASD ever undertaken in any community to date, there are limitations to the third study. First, the active consent process has degraded slightly over time. Parents providing permission for their children to participate was lower this time, 98.2% in wave 1, 93.6% in wave 2, and 80.7% in this wave. But the consent rate remains higher than elsewhere in a similar study with active consent protocols in any other population. For example, in the Clarren et al. (2001, p. 3) in-school study in Washington State where active consent was required, only “about 25%” of the parents allowed their children to participate. In Italy consent was obtained from 50% (May, et al., 2006). Also speaking to the completeness of this study, only 2 children suspected as cases of FAS or PFAS were lost to migration or refusal. A second weakness is that FAS and PFAS, even at the high rate detected, does not provide enough cases to perform meaningful multivariate analyses to more specifically sort out the interaction between prenatal alcohol exposure and: various demographic traits, childbearing and other maternal traits, environment, and other child psycho-social characteristics. Combining the results from multiple waves of research in this community will prompt and permit more sophisticated analyses. Third, some would say that retrospective maternal risk data is a weakness. But there is a need to balance the accuracy of diagnosis of the child (which is maximized by exams at age 6) vs the need for prospective data collected in prenatal clinics. Furthermore, while data collected in prenatal clinics is generally more complete (fewer missing data points) for low risk women, and the data may speak to the specific timing of exposure (e.g. weeks of gestation), it is often underreported. Actually, several studies have found that significantly higher rates of prenatal alcohol and substance abuse are reported retrospectively than prospectively (Alvik, et al., 2006; Czarnecki, et al., 1990). In our methods we build our alcohol questions on a context of nutrition, and working from daily reporting of current drinking in the past week and moving to prenatal daily drinking reports, we attempt to ensure accuracy. A fourth weakness of this study is that the FASD rates found in SA have limited comparability to developed or highly underdeveloped populations. This particular region in SA remains unique in culture and character from others in the world. The WCP Winelands remain as an “in between” population with second and third world conditions existing within parts of the country. But much of the WCP also has first world infrastructure and living conditions. However, the study of this community remains valuable for providing this community with specific information for their use in prevention, and for the general theoretical study of FASD. The methods used here can also be used elsewhere. The rate of FASD in this community remains a “worst case, highest rate scenario” for examining population-based etiological factors for FASD. Fifth, another possible weakness of this study is that we have not diagnosed the full spectrum of the IOM-defined categories of FASD. No study to date has reported on the full spectrum of FASD diagnoses (FAS, PFAS, ARBD, and ARND). This study, however, is the first in South Africa, and is among the first anywhere, to provide a complete breakdown and symptom-by-symptom detail of the two most severe diagnoses within the spectrum, FAS and PFAS. Most clinicians familiar with the diagnosis of FASD know that there are clearly distinguishing and varying levels of damage between cases of FAS meeting the IOM diagnostic criteria for FAS, some being immediately recognized as very severe and others being less clear cut from dysmorphology alone. This study documents efforts to utilize the IOM criteria that distinguish between the two most severe levels of FASD, FAS and PFAS. We believe these distinctions are important, especially in a binge drinking population. When using these two diagnoses in this South African population, a spectrum of effects emerges.
4.2 Methodological Considerations
Our methods of epidemiological research have improved. We are now quite familiar with this area and population. Using an operational version of the revised IOM methods is practical, consistent, reliable, and produces highly-specific diagnoses from examining all domains of variables (physical, psychological/developmental, and alcohol exposure). The dysmorphology values and scores are consistently quantified, allowing comparison and correlation of variables across domains. Active outreach in the schools ensures that selectivity and or omission is minimized. Cataloguing of all physical and psychological traits of the children and maternal drinking practices provides the opportunity for comparing FASD across populations for examinations of relative risk. Using similar methods and techniques in schools across populations would solve many of the problems of determining prevalence. Because of different active and passive measures utilized in various studies, there is little or no valid way to compare across studies.
4.3 Rates Over Time in this Community and Limited Comparisons
The rate of FAS remains high in this community, and the pattern of an excess of FASD births in the rural areas continues. The rate of FAS and PFAS combined among the first grade children in this wave of research (72.1 per 1,000) is 3.9% higher than the similar cohort rate from the wave 2 rate, 69.4 per 1,000, three years earlier (Viljoen, et al., 2005). However, since the consent to participate was lower this wave than in the past, the upper estimate per students screened is substantially higher than in the past (89.2 vs. 74.5, or 19.7% higher than three years earlier). Until substantial improvements in SES are made, or a massive comprehensive prevention program is instituted successfully to change specific social conditions that currently encourage heavy episodic maternal drinking (e.g. poverty, lack of education, boredom, and racial discrimination), then the FASD rate may well remain high.
Only two studies published among other populations can be directly and accurately compared to this population. In the only in-school study of FAS in the United States, Clarren, et al. (2001) used similar methods to find FAS in 3.1 per 1,000 first grade children. In a recent study in Italy using virtually identical methods among first grade students in randomly-selected schools, FAS was 3.7 to 7.4 per 1,000 and all FASD (FAS, PFAS, and one case of ARND) was found to be 20.3 to 40.5 per 1,000 (May, et al., 2006). Therefore, rates of FAS and FASD may be higher in all populations than has been indicated by clinic-based studies and other passive methodologies (Abel and Sokol, 1987; Abel, 1998; Sampson, et al., 1997) which report FAS as 0.5 to 2.0 per 1,000 and FASD at 8.7 per 1,000.
4.4 Alcohol Consumption Pattern as a Risk Factor
From this wave of research we have again found that binge drinking on weekends is still the pattern that causes most FASD, especially in the rural areas. Low SES and poverty in a society of extreme SES contrasts persist as risk factors (Crome and Glass, 2000). The severity of dysmorphology and the intelligence and behavioral characteristics of various levels of FASD are correlated with a variety of maternal drinking measures in this high poverty setting where many of the mothers are also small and suffer from poor nutrition, low education, a general powerlessness, and other related risk factors (May, et al., 2005; Viljoen, et al., 2002). On the positive side, a high percentage of the mothers of children with FAS reported much lower levels of current drinking (at time of interview) than during the index pregnancy or than reported in previous waves of research in this town: 12.2 drinks per week at interview for drinkers in Wave I (May, et al., 2000), 15.2 in Wave II (Viljoen, et al., 2005), and 7.8 in Wave III. However, one finding in this study begs for more attention. The amount of current drinking per week reported by the 24% of the controls who do drink is higher (8.9) than in previous research in this community. Even though the drinking of the control group drinkers is less concentrated on the weekends, and therefore probably less likely to be of a binge nature, it is of concern to the health of future children. Thus, while 76% of the controls report not drinking during past pregnancies, health care providers and prevention personnel should be vigilant with this trend in approximately one quarter of the maternal population.
4.5 Prevention
We are hopeful that the research carried out and the reporting back of results to parents and community constituents are lowering the rate of maternal drinking in this community. FASD are theoretically 100% preventable. Certainly a number of coalitions for the prevention of FAS have been formed among local health care providers, social service workers, and citizens since the initiation of research in this area, their activity being considerable at times. This community, and others like it, remains ripe for an efficacy trial of one or several types of prevention. Comprehensive approaches (Stratton, et al., 1996; May, 1995) utilizing universal education and broad behavioral change techniques are beginning, but will benefit from an infusion of resources and consistent organizational support. Specific, targeted prevention, using selective approaches such as screening all women attending prenatal clinics, are needed. And indicated prevention, utilizing case management for maternal support and guidance in a poorly-educated population could begin. For many individuals, a dual approach which encourages both drinking cessation (and reduction), and birth control for those who are not able to quit drinking (Masis and May, 1991), are promising. A comprehensive prevention trial utilizing these approaches has recently been funded by NIAAA to combat the high rate of FASD in this community.
5.0 CONCLUSIONS
The third wave of study in this region of South Africa has demonstrated that these methods of epidemiological research are consistent and improving in ability to diagnose at least two levels of FASD. The specific childhood features of FAS in this first grade population have been detailed as research and diagnostic methods applicable to similar studies in other populations. Similar methods can be used for accurate comparison of FASD prevalence and relative risk. The rate of FAS and Partial FAS remain at world record rates in this population, making this locale a prime candidate for further research into the etiology of FASD and for prevention programs to test the efficacy of FASD prevention.
ACKNOWLEDGMENTS
This project was funded by the National Institute on Alcohol Abuse, and Alcoholism (NIAAA) with supplements to grants #RO1 AA09440 and R01 AA11685, the National Institute on Minority Health and Health Disparities (NIMHD), and the Foundation for Alcohol Related Research (FARR) of South Africa. Faye Calhoun, D.P.A., Kenneth Warren, Ph.D., and T-K Li, M.D. of NIAAA helped initiate and have provided intellectual guidance, participated in, and supported the South African studies of FAS in a variety of ways since 1995. Our deepest thanks are extended to: Mayor Herman Bailey of the Western Cape community and the Town Council; Cecil Driver, Les Arendse, Japie Adams, Hilton Bailey, Henry Opperman, and the other principals and the staffs of the primary schools; and to many in the study community who graciously hosted and assisted in the research process. Phyllis Trujillo, David Buckley, Marita Brooks, Diana Baumgardner, and Kathy Deeshchii’nii of New Mexico have supported this project in manuscript preparation, administrative, and logistical matters. Maggie September, Debbie Price, Dickie Naude, and other colleagues and friends in the Republic of South Africa have participated with dedication and energy in the data collection and local research process.
Protocols and consent forms were approved by: The University of New Mexico (UNM) Medical School, (HRRC 96-209), the UNM College of Arts and Sciences (01-93-86-9908), the Research Ethics Committee of the University of Cape Town, The Office for Human Research Protection (OHRP) of NIH, and also a single-site assurance committee. Active consent for children to participate in all phases of this study was obtained from parents or other legal guardians. Mothers interviewed also provided a separate consent to participate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aase JM. Clinical recognition of FAS: difficulties of detection and diagnosis. Alcohol Health Res. World. 1994;18:5–9. [PMC free article] [PubMed] [Google Scholar]
- Aase JM, Jones KL, Clarren SK. Do we need the term “FAE”. Pediatrics. 1995;95:428–430. [PubMed] [Google Scholar]
- Abel EL. An update on the incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol. Teratol. 1995;17:43. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Abel EL. Fetal Alcohol Abuse Syndrome. Plenum Press; New York: 1998. [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in Fetal Alcohol Syndrome: provocative and permissive influences. Neurotoxicol. Teratol. 1995;17:445–465. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Abel EL, Sokol RJ. Incidence of Fetal Alcohol Syndrome and economic impact of FAS related anomalies. Drug Alcohol Depend. 1987;19:51–70. doi: 10.1016/0376-8716(87)90087-1. [DOI] [PubMed] [Google Scholar]
- Abel EL, Sokol RJ. A revised conservative estimate of the incidence of Fetal Alcohol Syndrome and its economic impact. Alcohol Clin. Exp. Res. 1991;15:514–524. doi: 10.1111/j.1530-0277.1991.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Alderton DL, Larson GE. Dimensionality of Raven’s advanced progressive matrices items. Educ. Psychol. Measurement. 1990;50:897–900. [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of Fetal Alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Adnams CM, Kodituwakku P, Hay A, Molteno C, Viljoen D, May PA. Patterns of Cognitive-Motor Development in Children with FAS from a Community in South Africa. Alcohol Clin. Exp. Res. 2001;25:557–562. [PubMed] [Google Scholar]
- Alvik A, Haldorsen T, Groholt B, Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin. Exp. Res. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Armstrong EM. Conceiving Risk, Bearing Responsibility: Fetal Alcohol Syndrome and the Diagnosis of Moral Disorder. The Johns Hopkins Press; Baltimore, Maryland: 2003. [Google Scholar]
- Barclay GW. Techniques of Population Analysis. John Wiley & Sons, Inc.; New York: 1958. [Google Scholar]
- Bishop DVM. Test for the reception of grammar: TROG. 2nd ed. University of Manchester; UK.: 1989. Published by the author and available from Age and Cognitive Performance Research Centre. [Google Scholar]
- Blalock HM., Jr. Social Statistics. 2nd ed. McGraw-Hill Book Company; New York: 1972. [Google Scholar]
- Burd L, Martsolf JT, Klug M. Children with Fetal Alcohol Syndrome in North Dakota: a case control study utilizing birth certificate data. Addict. Biol. 1996;1:181–189. doi: 10.1080/1355621961000124806. [DOI] [PubMed] [Google Scholar]
- Chavez GF, Cordero JF, Becera JE. Leading major congenital malformations among minority groups in the United States, 1981-1986. Morb. Mortal. Wkly. Rep. 1988;37:17–14. [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderson M, Fineman RM. Screening for Fetal Alcohol Syndrome in primary schools: a feasibility study. Teratology. 2001;63:3–10. doi: 10.1002/1096-9926(200101)63:1<3::AID-TERA1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Crome IB, Glass,Y. The. Dop System: a Manifestation of Social Exclusion. A Personal Commentary on “Alcohol Consumption Amongst South African Workers,” by L. London. Drug Alcohol Dep. 1999;59:207–208. doi: 10.1016/s0376-8716(99)00121-0. [DOI] [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D. Five -year reliability of self-reported alcohol consumption. J. Stud. Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- Dean AG, Dean JA, Coulombier D, et al. Epi Info™, Version 6.04, A Word Processing, Database, and Statistics Program for Public Health on IBM-compatible Microcomputers. Centers for Disease Control and Prevention; Atlanta: 1996. [Google Scholar]
- Duimstra C, Johnson D, Kutsch C, Wang B, Zentner M, Kellerman S. A fetal alcohol syndrome surveillance pilot project in American Indian communities in the Northern Plains. Public Health Rep. 1993;108:225–229. [PMC free article] [PubMed] [Google Scholar]
- Egeland GM, Perham-Hester KA, Gesaner BO, Ingle D, Berner JE, Middaugh JP. Fetal Alcohol Syndrome in Alaska, 1977-1992: an administrative prevalence service from multiple sources. Am. J. Pub. Health. 1998;88:781–786. doi: 10.2105/ajph.88.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland GM, Perham-Hester KA, Hook EB. Use of capture-recapture analyses in Fetal Alcohol Syndrome surveillance in Alaska. Am. J. Epidemiol. 1995;141:335–341. doi: 10.1093/aje/141.4.335. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller J, Aragon AA, Khaole N, Viljoen DL, Jones KL, Robinson LK. A Practical Clinical Approach to Diagnosis of Fetal Alcohol Spectrum Disorders: clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . In: Fetal Alcohol Syndrome diagnosis, epidemiology, prevention, and treatment. Stratton Kathleen, Howe Cynthia, Battaglia Fredrick., editors. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal alcohol exposure and neurobehavioral development: where is the threshold. Alcohol Clin. Exp. Res. 1994;18:30–36. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol. Teratol. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the Fetal Alcohol Syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. An alternative to standard drinks as a measure of alcohol consumption. J. Subs. Abuse. 2000;12:67–78. doi: 10.1016/s0899-3289(00)00042-0. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am. J. Pub. Health. 1994;84:294–296. doi: 10.2105/ajph.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Flisher AJ, Noubary F, Reece R, Marais A, Lombard C. Substance abuse and behavioral correlates of sexual assault among South African adolescents. Child Abuse Negl. 2004;28:683–696. doi: 10.1016/j.chiabu.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Kvigne V, Leonardson G, Neff-Smith M, Brock E, Borzelleca J, Welty T. Characteristics of children who have full or incomplete Fetal Alcohol Syndrome. Pediatrics. 2004;145:635–640. doi: 10.1016/j.jpeds.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Harouseau H, Borteryn JT, Menuet JC. Les enfants de parents alcooliques: anomalies observées á propos de 127 cas. Quest. Medical. 1968;21:476–482. [Google Scholar]
- Little BB, Snell LM, Gilstrap LCI, Gant NF. Failure to recognize Fetal Alcohol Syndrome in newborn infants. Am. J. Dis. Child. 1990;144:1142–1146. doi: 10.1001/archpedi.1990.02150340088030. [DOI] [PubMed] [Google Scholar]
- London L. Alcohol Consumption Amongst South African Farm Workers: a Challenge for Post-apartheid Health Sector Transformation. Drug Alcohol Depend. 2000;59:199–226. doi: 10.1016/s0376-8716(99)00120-9. [DOI] [PubMed] [Google Scholar]
- London L, Meyers J, Nell V, Taylor T, Thompson ML, Milbuli SS. An investigation into the neurological and neurobehavioral effects of long term agrochemical exposure among deciduous fruit farm workers in the Western Cape, South Africa. University of Cape Town; 1995. M.D. Thesis. [DOI] [PubMed] [Google Scholar]
- Mager A. White liquor hits black lives: meaning of excessive liquor consumption in South Africa in the second half of the twentieth century. Soc. Sci. Med. 2004;59:735–751. doi: 10.1016/j.socscimed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Masis KB, May PA. A comprehensive local program for the prevention of fetal alcohol syndrome. Public Health Rep. 1991;106:484–489. [PMC free article] [PubMed] [Google Scholar]
- May PA. Fetal alcohol effects among North American Indians. Alcohol Health Res. World. 1991;15:239–248. [Google Scholar]
- May PA. A multiple-level, comprehensive approach to the prevention of FAS and other alcohol-related birth defects. Int. J. Addict. 1995;30:1549–1602. doi: 10.3109/10826089509104417. [DOI] [PubMed] [Google Scholar]
- May PA. Research issues in the prevention of Fetal Alcohol Syndrome and alcohol-related birth defects. In: Howard J, Martin S, Mail P, Hilton M, Taylor E, editors. Women and Alcohol: issues for Prevention Research. 1996. pp. 93–131. NIAAA Research Monographs 32. [Google Scholar]
- May PA, Brooke LE, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson LK, Viljoen D. The epidemiology of Fetal Alcohol Syndrome in a South African community in the Western Cape Province. Am. J. Pub. Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke LE, Gossage JP, Snell C, Hendricks L, Croxford J, Marais AS, Viljoen DL. Maternal risk factors for Fetal Alcohol Syndrome in the Western Cape Province of South Africa: a Population-based study. Am. J. Pub. Health. 2005;95:90–199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, Kalberg WO, Hoyme HE, Robinson LK, Coriale G, Jones KL, Del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M. The epidemiology of FASD in a province in Italy: prevalence and characteristics of children in a random sample of schools. Alcohol Clin. Exp. Res. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of Fetal Alcohol Syndrome: a summary. Alcohol Res. Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Hymbaugh KJ, Aase JM, Samet JM. Epidemiology of Fetal Alcohol Syndrome among American Indians of the Southwest. Soc. Biol. 1983;30:374–385. doi: 10.1080/19485565.1983.9988551. [DOI] [PubMed] [Google Scholar]
- May PA, McCloskey J, Gossage JP. Fetal alcohol syndrome among American Indians: epidemiology, issues, and research. In: Mail PD, Heurtin-Roberts S, Martin SE, Howard J, editors. Alcohol Use Among American Indians: Multiple Perspectives on a Complex Problem. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD.: 2002. pp. 321–369. National Institute on Alcohol Abuse and Alcoholism Research Monograph No. 37. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Fetal Alcohol Syndrome: report on the Site Visit to South Africa. Rockville, MD.: 1996. NIAAA Report. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Fetal Alcohol Syndrome: South Africa, A Progress Report on the 199 7 Pilot Study, Information Exchange, and Prevention Workshop. Rockville, MD.: 1998. NIAAA Report. [Google Scholar]
- Palmer C. Fetal alcohol effects - incidence and understanding in the Cape. S. Afr. Med. J. 1985;68:779–80. [PubMed] [Google Scholar]
- Parry CDH, Bennetts AL. Alcohol Policy and Public Health in South Africa. Oxford University Press; Cape Town: 1998. [Google Scholar]
- Quaid J, Kirkpatrick J, Nakamura R, Aase JM. Establishing the occurence of FAS/FAE in a rural community. The Provider. 1993;18:71–75. [Google Scholar]
- Republic of South Africa . 1996 Census of the Population. Bureau of Census; Pretoria: 1997. [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas BS, Ziolkowski M. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst. Use Misuse. 2001;36:313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinker’s reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sokol RF, Clarren SK. Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring. Alcohol Clin. Exp. Res. 1989;13:597–598. doi: 10.1111/j.1530-0277.1989.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC. Fetal alcohol syndrome diagnosis, epidemiology, prevention, and treatment. Institute of Medicine (Division of Biobehavioral Sciences and Mental Disorders, Committee to Study Fetal Alcohol Syndrome and National Institute on Alcohol Abuse and Alcoholism) National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Press S, Sampson PD. A fetal alcohol behavior scale. Alcohol Clin. Exp. Res. 1998;22:325–333. doi: 10.1111/j.1530-0277.1998.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Clarren SK, Jones KL. Natural history of the Fetal Alcohol Syndrome, A ten-year follow-up of eleven patients. Lancet. 1985;2:85–92. doi: 10.1016/s0140-6736(85)90189-8. [DOI] [PubMed] [Google Scholar]
- Sullivan WC. A note on the influence of maternal inebriety on the offspring. J. Mental Sci. 1899;45:489–503. doi: 10.1093/ije/dyr006. [DOI] [PubMed] [Google Scholar]
- Unpublished Vital Statistics from the studied community, no date.
- Viljoen DL, Craig P, Hymbaugh K, Boyle C, Blount S. Fetal Alcohol Syndrome - South Africa, 2001. Morb. Mortal. Wkly. Rep. 2003;52:660–662. [PubMed] [Google Scholar]
- Viljoen DL, Croxford J, Gossage JP, May PA. Characteristics of mothers of children with Fetal Alcohol Syndrome in the Western Cape Province of South Africa: a case control study, J. Stud. Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole N, Asante KK, Findlay R, Quinton B, Brooke LE, May PA. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J. Stud. Alc. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]