Abstract
Purpose
To reduce cardiotoxicity from breast radiotherapy (RT), innovative techniques are under investigation. Information about cardiac motion with respiration and positional reproducibility under active breathing control (ABC) is necessary to evaluate these techniques.
Methods
Patients requiring loco-regional RT for breast cancer were CT-scanned using an ABC device at various breath-hold states, before and during treatment. Ten patients were studied. For each patient, 12 datasets were analyzed. Mutual information-based regional rigid alignment was used to determine the magnitude and reproducibility of cardiac motion as a function of breathing state. For each scan session, motion was quantified by evaluating the displacement of a point along the left anterior descending artery (LAD) with respect to its position at end expiration. Long-term positional reproducibility was also assessed.
Results
Displacement of the LAD was greatest in the inferior direction, moderate in the anterior direction, and lowest in the left-right direction. At shallow breathing states, the average displacement of LAD position was up to 6mm in the inferior direction. The maximum displacement in any patient was 2.8cm in the inferior direction, between expiration and deep-inspiration breath hold (DIBH). At end expiration, the long-term reproducibility (SD) of the LAD position was 3mm in the A-P, 6mm in the S-I, and 4mm in the L-R directions. At DIBH, long-term reproducibility was 3mm in the A-P, 7mm in the S-I, and 3mm in the L-R directions.
Conclusions
These data demonstrate the extent of LAD displacement that occurs with shallow breathing and with DIBH. This information may guide optimization studies considering the effects of respiratory motion and reproducibility of cardiac position on cardiac dose, both with and without ABC.
Keywords: radiation planning, breast cancer, heart, respiratory motion, active breathing control
INTRODUCTION
Adjuvant radiation therapy (RT) is an integral component of breast cancer treatment.1,2 However, several studies have suggested increased risks of mortality from ischemic heart disease in breast cancer patients treated with radiation, particularly to the left side of the chest.1,2,3,4,5,6,7 While there are some indications that the increased risk of ischemic heart disease associated with radiation therapy has decreased as target definition and treatment techniques have improved over time,8 cardiac perfusion defects have been documented even in patients treated from 1998-2002 with three-dimensional planning.9
Further innovations in treatment planning are therefore under investigation, including intensity modulation10,11,12 and various techniques for the control of respiratory motion.13,14,15,16,17,18,19,20,21 The aim of these techniques is to reduce still further the dose received by the heart, in the hopes of minimizing the long-term morbidity and mortality associated with the treatment of breast cancer.
The ability to compare and evaluate treatment plans generated by different planning techniques depends critically upon access to a sophisticated model of the patient, including an understanding of the effects of respiratory motion upon organ position. Accurate information about respiratory motion is necessary not only regarding target structures, in order to define appropriate margins, but also regarding critical normal tissues, in order to allow optimization of treatment planning studies and minimization of treatment-related toxicities. As Figure 1 depicts, motion of the heart with respiration may be quite substantial in some patients. Furthermore, because of the growing interest in breathing-controlled radiation, an understanding of positional reproducibility in breath-hold states is necessary to determine the appropriate role of breathing control in breast cancer treatment. After all, which of these techniques has greatest promise for reducing cardiac morbidity depends critically upon the nature of cardiac organ motion during the respiratory cycle, as well as upon the reproducibility of cardiac position during breath-hold states.
Figure 1.
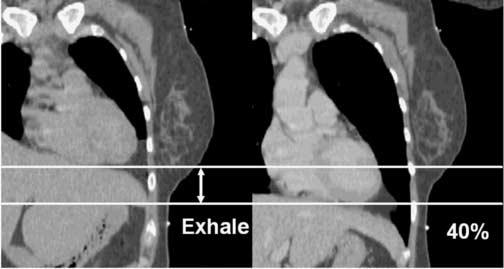
Heart Motion with Respiration
Qualitatively, heart motion with respiration appears to be substantial in some patients.
METHODS
Patients at the University of Michigan Medical Center who required loco-regional RT for breast cancer treatment were asked to consent to participate in an IRB-approved protocol to acquire a series of CT scans performed at various breath-hold states. All patients had histologically confirmed adenocarcinoma of the breast, AJCC Stage T0-3, N0-3, M0, and had undergone either lumpectomy or mastectomy.
Patients were scanned utilizing a high-speed 16-slice CT scanner in the treatment position on a flat table top. Scan duration was approximately 10 seconds to obtain images from the low neck through thorax (of which approximately 3 seconds were required to obtain images in the region of the heart and great vessels). An ABC device (vMax, Sensormedics, Yorba Linda, CA) was used to control breath hold at end expiration, as well as 20%, 40%, and 80% of vital capacity (VC, as assessed at the time of the treatment planning scan). Scans were performed prior to treatment (for planning) and after approximately 1/3 and 2/3 of treatment (for reproducibility assessment). While respiration was controlled, cardiac contraction continued freely, without EKG-gating, such that the beat-to-beat motion of the heart affected the images acquired (a free-beating, but not free-breathing, scan). Given the scan duration and the normal rate of cardiac contraction, three to four cardiac contractions would have occurred during the course of image acquisition in the region of interest for most patients, and therefore the beating motion of the heart was expected to introduce a complex but subtle sampling artifact that was not controlled in any way and should be incorporated into the error of the measurements.
For this study, we utilized information obtained from the first ten sequential patients enrolled in the study whose scans were adequate for analysis. For each patient, a total of 12 datasets were acquired and analyzed, where each dataset consisted of approximately 140 images, with slice thickness of 2.5 mm.
All scans were imported into the University of Michigan treatment planning system, UMPlan. A physician contoured the heart on the exhale scan and deep inhale (at 80% of VC) scans, the scans which would serve as the source for the reference datasets used in the mutual information-based rigid regional alignment process to follow. The heart contour was then asymmetrically expanded (5 cm superiorly and 1 cm in the axial plane and inferiorly) to allow inclusion of the great vessels and surrounding tissues in the reference dataset in order to provide additional relevant anatomical features for alignment. Registration software developed at the University of Michigan was used for the alignment.22 To aid in initialization of the alignment, a physician manually identified three pairs of homologous points: one at the bifurcation of the pulmonary trunk, one at the right mid-aspect of the left ventricular outflow tract, and one at the anterior-most point of the left anterior descending (LAD) coronary artery. The LAD point is depicted in Figure 2. The alignment software iteratively modified the parameters of the rigid body transformation on the test dataset (all other breathing states were aligned to the exhale state). Using mutual information as a metric, the optimal transformation was determined iteratively. The transformation described the extent of regional motion. Alignments were then visually reviewed in all planes. Figure 3 shows the outcome of the registration process in a typical patient on the coronal and sagittal planes.
Figure 2.
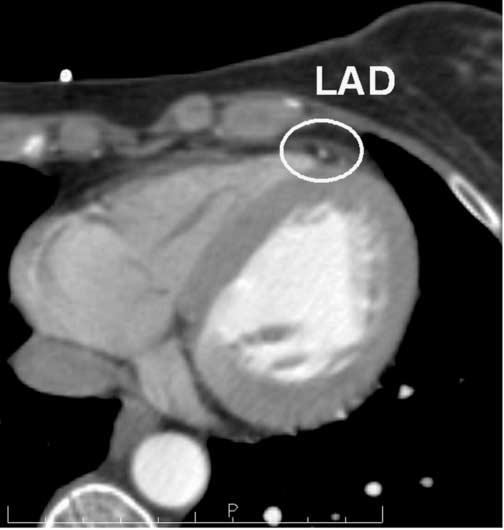
The Anterior-Most Point of the Left Anterior Descending Coronary Artery
The anterior-most point of the left anterior descending artery (LAD) was defined on all scan sets, as depicted here.
Figure 3.
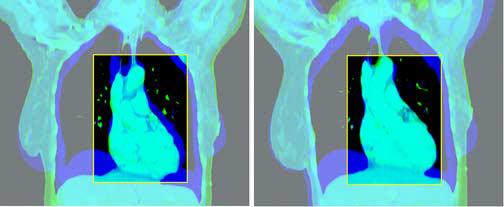
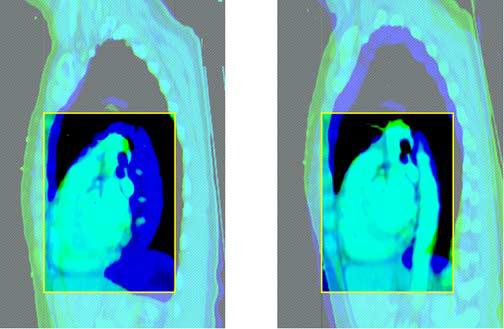
Rigid Regional Alignment (this figure is in 2 2-part panels, each in its own file: the first panel should be subtitled “Coronal”, with the first part labelled “Before” and the second part labelled “After”; the second panel should be subtitled “Sagittal”, with the first part labelled “Before” and the second part “After”).
This figure depicts the outcome of the rigid regional alignment process. Here, a patient's scan at 80% of vital capacity was aligned to her scan in the exhale state from the same scan session.
Table 1 describes the reference and evaluated datasets for both short-term and long-term evaluation of the cardiac motion. The magnitude of the cardiac motion was defined as the displacement of the anterior-most point of the left anterior descending artery (LAD) from the test (20-80% VC) to the exhale state during that same scan session. For each breath-hold state (20, 40, and 80%), the average intra-fraction motion and standard deviation of the LAD motion was determined for all patients.
Table 1.
Description of Experiments to Assess Short-Term Motion and Reproducibility and Long-Term Reproducibility of the Left Anterior Descending Artery of the Heart using Active Breathing Control
| Experiment | Reference Dataset | Comparison Datasets |
Alignment Information |
|---|---|---|---|
| Short-term motion and reproducibility |
CT Session #1 Exhale |
Session #1 at 20, 40, and 80% of VC |
ROI alignment around heart |
| CT Session #2 Exhale |
Session #2 at 20, 40, and 80% of VC |
ROI alignment around heart |
|
| CT Session #3 Exhale |
Session #3 at 20, 40, and 80% of VC |
ROI alignment around heart |
|
| Long-term reproducibility |
CT Session #1 Exhale |
Session #2 Exhale |
|
| Session #3 Exhale | |||
| CT Session #1 80% VC |
Session #2 80% |
|
|
| Session #3 80% |
Long-term (inter-fraction) reproducibility of the position of this LAD point was also assessed as described in Table I. For these assessments, first, the spine was registered for the exhale datasets of session 2 and 3 to the session 1 exhale dataset to ensure skeletal alignment and thereby remove most effects of differences in patient positioning on the scanning table during the different sessions. Subsequently, the region of the heart was aligned for the same datasets, using the three points placed in the cardiac region as described above to provide a starting place for the alignment, and the mean displacement and standard deviation (SD) of the reference LAD point was assessed after correction for the spine-to-spine alignment between exhale datasets. The same technique was applied to examine long-term (inter-fraction) reproducibility for the 80% datasets with the first session 80% dataset as the reference set.
RESULTS
Of the ten patients whose scans were utilized in this study, 4 had undergone mastectomy and 6 lumpectomy. Median patient age was 49 (minimum 39, maximum 61). All patients were female.
As shown in Table 2, the LAD point was displaced in the anterior and inferior directions with inspiration; the magnitude of displacement from the expiratory position increased as inspiration deepened. Displacement of the LAD was greatest in the inferior direction, moderate in the anterior direction, and lowest in the left-right direction.
Table 2.
Motion of the Left Anterior Descending Artery of the Heart Based on Repeat CT Scans with Active Breathing Control in 10 Patients
| Displacement (mm) | ||||||
|---|---|---|---|---|---|---|
| Breathing state |
Anterior- Posterior |
Standard Deviation |
Inferior- Superior |
Standard Deviation |
Left-Right | Standard Deviation |
| 20 | 0.01 ant | 2.4 | 0.76 inf | 6.1 | 0.30 left | 2.7 |
| 40 | 2.83 ant | 3.0 | 5.78 inf | 7.6 | 1.59 right | 4.4 |
| 80 | 7.04 ant | 4.0 | 11.77 inf | 7.1 | 2.58 right | 4.9 |
At shallow breathing states reflective of tidal breathing (up to 40% of VC), the average displacement of LAD position was 5.8 mm in the inferior direction, 2.8 mm in the anterior direction, and 1.6 mm in the right direction. The maximum displacement in any one patient in the inferior direction was 2.54 cm for shallow breathing states and 2.84 cm for the DIBH (80% VC) state. The maximum displacement in any one patient in the anterior direction was 0.92 cm for shallow breathing states and 1.55 cm for the DIBH state. The maximum displacement in any one patient in the right direction was 1.26 cm for shallow breathing states and 1.32 cm for the DIBH state.
At end expiration, the long-term reproducibility (SD) of the LAD position was 6.0 mm in the S-I, 3.3 mm in the A-P, and 4.5 mm in the L-R directions. In the DIBH state, the long-term reproducibility was 6.8 mm in the S-I, 3.4 mm in the A-P, and 2.7 mm in the L-R directions.
DISCUSSION
Motivated in large part by concerns about potential cardiotoxicity, a variety of methods for achieving greater dose conformality in breast radiotherapy are actively being explored. The proximity of the heart and its left anterior descending artery—the coronary vessel that supplies a usually critical region of myocardium—to the targets of breast radiotherapy, including the chest wall and the internal mammary lymphatics, poses a difficult treatment planning challenge in some patients. Respiratory motion of the heart adds an additional layer of complexity to this challenge, insofar as breathing may cause parts of this critical normal tissue to move into and out of even the most highly conformal radiation fields, as suggested by Figure 3. Therefore, detailed information regarding the precise nature of the respiratory motion of the heart is necessary to guide the selection of the techniques most likely to spare this critical normal structure.
Movement of the LAD in the left-right and anterior-posterior directions is most likely to be clinically relevant because of the typical size and orientation of breast radiotherapy fields and the length, quasi-linear shape, and roughly longitudinal orientation of the LAD itself. Moderate superior or inferior displacement of the LAD is unlikely to change substantially the volume of that structure receiving dose. In this study, we document relatively small average displacement of the LAD in the left-right and anterior-posterior directions with free breathing, but more substantial displacement in individual patients.
These data are important for determining a more accurate estimate of the delivered dose distributions than that calculated by treatment planning systems on a single CT scan dataset obtained during free breathing. After all, if free breathing causes substantial motion, dose actually delivered to the LAD may be higher or lower than that calculated, with resulting implications for biologic modeling of dose-response and the probability of normal tissue complications. Our results suggest that motion in the most clinically relevant directions with free breathing is, on average, relatively limited, but that it may be considerable in individual patients. With increasing conformality of treatment, the effects of respiratory motion upon the doses received by both target and normal tissues become more significant. Therefore, a variety of strategies to compensate for breathing motion have been considered. These include gated irradiation, the use of abdominal pressure, voluntary shallow breathing, voluntary deep inspiration, and treatment under active breathing control.13-21.
Active breathing control is a particularly intriguing approach, in which a computer-controlled valve closes the inhalation and exhalation paths at a predetermined point in the respiratory cycle (monitored by computer integration of measured flow), causing a controlled breath hold, so that treatment may be accomplished during a period in which breathing is temporarily suspended. In order to assess the merits of the active breathing control approach, however, it is necessary to obtain a sophisticated understanding of the reproducibility of normal tissue position with that technique. After all, elimination of respiratory motion is not likely to yield substantial benefits if the error from non-reproducibility of normal tissue position is as great as the displacement that occurs during normal free breathing. If, however, breathing motion is significant, and breath-hold states yield reproducible positioning of critical normal structures, there may be a benefit from the use of active breathing control. For example, a study using active breathing control has demonstrated the advantage of breath hold techniques to minimize the dose to the heart.23 That study focused on a treatment plan evaluation rather than reproducibility of the heart positioning with ABC. In another study, the reproducibility of positioning of the lungs and carina was evaluated for breast cancer patients and found to vary between 1-2 mm depending on the location.24
The deep inspiration breath-hold position25 is particularly promising for treatment of breast cancer patients because it maximizes the distance between the heart and chest wall due to the expansion of the lungs. In this study, reproducibility of the LAD in the DIBH position in each direction was found to be on the order of the average displacement with free breathing.
Of note, this work used a rigid regional approximation to assess the magnitude of heart motion as a function of breath hold state. However, the heart is a flexible structure that itself changes shape with the changes in intrathoracic pressure that accompany respiration. Therefore, a more accurate approach would be to use a deformable registration technique, which we are currently developing and assessing. Nevertheless, visual inspection of the rigid alignments performed in this study suggests that the changes in cardiac shape with respiration were relatively small, particularly in the area of interest surrounding the LAD, and that the technique employed was reasonably accurate. Thus, this study provides useful information until a deformable registration technique becomes available.
In summary, these data demonstrate the extent of LAD displacement that occurs with shallow breathing and with deep inspiration, as well as the positional reproducibility of the LAD under active breathing control. This information is useful for guiding optimization studies that consider the effects of respiratory motion and reproducibility of cardiac position on the dose received by this critical normal structure, both with and without active breathing control.
Footnotes
Presented in oral form at the Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 2005 (Denver, Colorado).
This work was sponsored by NIH R01 CA102435-01 and P01 CA59827. The authors certify that this is original, unpublished work. We have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355(9217):1757–70. [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group Effect of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol. 1998;16:2625–31. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 4.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys. 1999;43:755–62. doi: 10.1016/s0360-3016(98)00412-x. [DOI] [PubMed] [Google Scholar]
- 5.Rutqvist LE, Lax I, Fornander T, et al. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–96. doi: 10.1016/0360-3016(92)90784-f. [DOI] [PubMed] [Google Scholar]
- 6.Rutqvist LE, Johansson H. Mortality by lateralty of the primary tumour among 55000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer. 1990;61:866–8. doi: 10.1038/bjc.1990.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–53. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 8.Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97(6):419–24. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–23. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Krueger EA, Fraas BA, McShan DL, et al. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56(4):1023–37. doi: 10.1016/s0360-3016(03)00183-4. [DOI] [PubMed] [Google Scholar]
- 11.Landau D, Adams EJ, Webb S, et al. Cardiac avoidance in breast radiotherapy: a comparison of simple shielding techniques with intensity-modulated radiotherapy. Radiother Oncol. 2001;60:247–55. doi: 10.1016/s0167-8140(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 12.Cho BCJ, Schwartz M, Mijnheer BJ, Bartelink H. Simplified intensity-modulated radiotherapy using pre-defined segments to reduce cardiac complications in left-sided breast cancer. Radiother Oncol. 2004;70:231–41. doi: 10.1016/j.radonc.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Kubo HD, Len PM, Minohara S, et al. Breathing-synchronized radiotherapy program at the University of California Davis Cancer Center. Med Phys. 2000;27(2):346–53. doi: 10.1118/1.598837. [DOI] [PubMed] [Google Scholar]
- 14.Uematsu M, Shioda A, Suda A, et al. Intrafractional tumor position stability during computer tomography (CT)-guided frameless stereotactic radiation therapy for lung of liver cancers with a fusion of CT and linear accelerator (FOCAL) unit. Int J Radiat Oncol Biol Phys. 2000;48(2):443–8. doi: 10.1016/s0360-3016(00)00619-2. [DOI] [PubMed] [Google Scholar]
- 15.Hanley J, Debois MM, Mah D, et al. Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys. 1999;45(3):603–11. doi: 10.1016/s0360-3016(99)00154-6. [DOI] [PubMed] [Google Scholar]
- 16.Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51(5):1410–21. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 17.Krauss DJ, Kestin LJ, Raff G, et al. MRI-based volumetric assessment of cardiac anatomy and dose reduction via active breathing control during irradiation for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2005;61(4):1243–50. doi: 10.1016/j.ijrobp.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49(1):199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen AN, Korreman S, Nystrom H, Specht L. Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol. 2004;72:53–60. doi: 10.1016/j.radonc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Lu HM, Cash E, Chen MH, et al. Reduction of cardiac volume in left-breast treatment fields by respiratory maneuvers: a CT study. Int J Radiat Oncol Biol Phys. 2000;47(4):895–904. doi: 10.1016/s0360-3016(00)00512-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen MH, Cash EP, Danias PG, et al. Respiratory maneuvers decrease irradiated cardiac volume in patients with left-sided breast cancer. J Cardiovasc Magn Reson. 2002;4(2):265–71. doi: 10.1081/jcmr-120003952. [DOI] [PubMed] [Google Scholar]
- 22.Kessler ML, Li K, Solack J, Archer P, Meyer C. Clinical Use of a Mutual Information-Based Automated Image Registration System for Conformal Radiotherapy Treatment Planning. Lecture Notes in Computer Science. 2001;2208:1255–57. [Google Scholar]
- 23.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 24.Remouchamps VM, Letts N, Yan D, Vicini FA, Moreau M, Zielinski JA, Liang J, Kestin LL, Martinez AA, Wong JW. Three-dimensional evaluation of intra- and interfraction immobilization of lung and chest wall using active breathing control: a reproducibility study with breast cancer patients. Int J Radiat Oncol Biol Phys. 2003 Nov 15;57(4):968–78. doi: 10.1016/s0360-3016(03)00710-7. [DOI] [PubMed] [Google Scholar]
- 25.Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49(1):199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]


