Abstract
Background
Considerable evidence suggests that indirect recognition of MHC allopeptides plays an important role in solid-organ rejection. Here, we examine whether immunization with class I or class II allopeptides accelerates rejection in a fully MHC-mismatched lung transplant model in miniature swine.
Methods
Recipients were immunized with either donor-derived class I or class II peptides. Sensitization to the peptides was confirmed by DTH testing and in vitro proliferation assays. Non-immunized control (n=6), class I peptide-immunized (n=3) and class II peptide-immunized (n=3) swine were transplanted with fully mismatched lungs using only a 12-day course of tacrolimus.
Results
One control animal rejected its graft on POD 103, while the others maintained their grafts over one year. In the class I peptide-immunized group, two recipients rejected their grafts (POD 14 and 52). The third animal has not rejected the graft (POD120, experiment is ongoing). In contrast, in the class II-peptide immunized group, only one animal rejected its graft on POD52, while the others maintained their grafts over one year. Both anti-donor IgM and IgG antibodies were detectable in all acute rejectors, although no alloantibody was detectable in long-term acceptors. Regardless of the fate of the graft, all animals have maintained their proliferative responses to the peptides. However, only acceptors maintained donor-specific hyporesponsiveness in cell-mediated lymphocytotoxity and mixed lymphocyte reaction assays.
Conclusions
Pre-transplant sensitization of lung allograft recipients to donor allopeptides accelerates graft rejection. This appears particularly true for class I-derived allopeptides, suggesting that class II molecules may be less antigenic when presented indirectly.
INTRODUCTION
It is generally accepted that there are two distinct pathways of allorecognition. In the direct pathway, T cells recognize intact allogeneic MHC molecules on the surface of donor antigen presenting cells. In the indirect pathway, T cells recognize processed alloantigen as peptides presented in the context of self MHC molecules. Large animal studies from our laboratory have shown that immunization with donor-derived class I allopeptide can accelerate the rejection of a graft in a cyclosporine-based class I mismatched heart2 or lung3 transplant rejection model. In this study, we examine the effect of allopeptide immunization in a tacrolimus-based, fully MHC-mismatched lung allograft model that is typically resistant to rejection.
MATERIALS AND METHODS
Three MHC class I allopeptides derived from the hypervariable regions of the swine leukocyte antigen (SLA) class Ic P14 α1 helix, and seven MHC class IIc allopeptides derived from the polymorphic β-1 domains of the SLA class IIc DR and DQ loci were synthesized. SLAdd swine were immunized with either the mixture of PC14 class I peptides or the mixture of DR and DQ class II peptides 21 days before transplantation. Sensitization to these peptides was confirmed by in vivo DTH testing and in vitro proliferation assays as previously described2. Transplant donors and recipients were selected from our herd of partially inbred miniature swine at 5–9 months of age. Non-immunized control swine (n=6), class I peptide-immunized swine (n=3) and class II peptide-immunized swine (n=3) were transplanted with two-haplotype fully MHC- mismatched orthotopic left lungs, and then treated with a 12-day course of tacrolimus (Fujisawa, Deerfield, IL. 0.15 mg/kg/day, as a continuous IV infusion; target level = 35 to 50 ng/ml). Orthotopic left lung transplantation was performed as previously described5. Lung allografts were monitored by physical examination, serial chest radiography and open lung biopsies. Graft loss (our principal endpoint) is defined as high-grade histologic rejection in association with loss of graft aeration as observed on chest radiograph, and/or loss of graft perfusion or compliance as observed intra-operatively at the time of open lung biopsy. Reactivity to the peptides was tested by in vitro proliferation assays and immune responses to donor cells were monitored with cell-mediated lympholysis (CML) and mixed lymphocyte reaction (MLR). Sera from animals were tested for the presence of anti-donor IgM and IgG antibodies by indirect flow cytometry.
RESULTS
Fourteen days after immunization, all swine showed reactivity to at least one of the donor-derived peptides by DTH testing, with some peptides being more immunogenic than others (Table. 1) Also, in vitro proliferative responses confirmed the presence of T cell reactivity to either class I or class II peptides, as appropriate. In the non-immunized control group, one animal rejected its graft on POD 103, while the other five recipients maintained their grafts over one year. In the class I peptide-immunized animals, two recipients rejected their grafts in 14 and 52 days in an accelerated fashion (Fig. 1), as compared to controls. The third animal has not rejected the graft (POD120, experiment is ongoing). In contrast, in the class II-peptide immunized animals, only one animal rejected its graft on POD52, while the other two recipients maintained their grafts over one year as same as the control animals. All rejectors had anti-donor IgM and IgG by the time of rejection. The class I-immunized recipient that still maintains its graft showed transient production of anti-donor IgM, but has never developed anti-donor IgG alloantibodies. In the control and class II immunized acute rejectors, anti-donor IgM and IgG antibodies were also detectable; however, no alloantibody was detectable among the long-term acceptors. Regardless of the fate of the graft, all animals have maintained their in vitro proliferative responses to the peptides. However, only acceptors maintained donor-specific hyporesponsiveness in CML and MLR assays.
Table 1.
In vivo DTH response in pigs immunized with donor-derived class Ic or IIc peptides
| (A) mm of induration in animals immunized with class Ic peptides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| animal | PC14-1 | PC14-2 | PC14-3 | MTB | RT1Du | ||||
| 16617 | 9 | 5 | 17 | 12 | 0 | ||||
| 16788 | 3 | 6 | 19 | 18 | 0 | ||||
| 16955 | 0 | 0 | 17 | 14 | 0 | ||||
| (B) mm of induration in animals immunized with class IIc peptides | |||||||||
|
| |||||||||
| animal | DR1 | DR2 | DR3 | DQ2 | DQ3 | DQ4 | DQ5 | MTB | RT1Du |
|
| |||||||||
| 16284 | 14 | 9 | 13 | 11 | 13 | 0 | 11 | 18 | 0 |
| 16486 | 12 | 7 | 10 | 5 | 8 | 0 | 5 | 15 | 0 |
| 16638 | 16 | 0 | 12 | 11 | 13 | 0 | 13 | 27 | 0 |
positive response: >10 mm
MTB : Mycobacterium tuberculosis H37 RA (positive control)
RT1Du: xenogeneic rat class II peptide (negative control)
Figure 1.
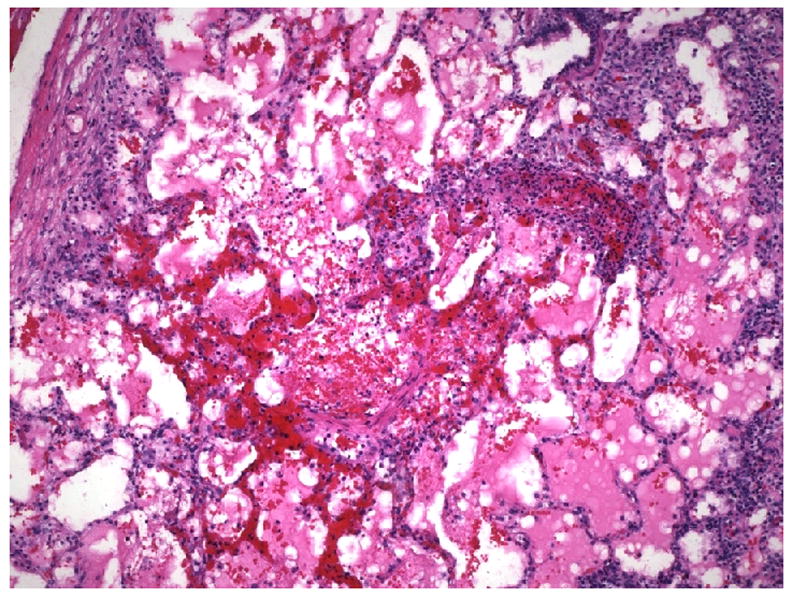
Histological findings of lung allograft from class I peptide-immunized, fully mismatched lung recipient on POD 14; severe mononuclear cell infiltration with necrosis and hemorrhage consistent with ISHLT grade 4/4 acute rejection (H&E staining, × 100).
CONCLUSIONS
This study was undertaken to establish the role of indirect allorecognition in a clinically relevant model of orthotopic lung transplantation. Our study demonstrates that pre-transplant sensitization of lung allograft recipients to donor-derived peptides accelerates graft rejection. This effect seems to be more pronounced following immunization with class I allopeptides, than with class II peptides. These data suggest that class II molecules may be less antigenic when presented indirectly. The maintenance of donor-specific cellular hyporesponsiveness among the long-term acceptors (in some cases despite sensitization to relevant allopeptides) implies that our 12-day treatment with high-dose tacrolimus has induced a state of strong immune regulation that is able to protect these fully mismatched grafts in the absence of further immunosuppression. Further studies are needed to characterize this putative regulatory effect.
Footnotes
This work was supported in part by grants from the Thoracic Surgery Foundation for Research and Education (Sahara/Allan), the International Society for Heart and Lung Transplantation (Shoji), the American Surgical Association Foundation (Allan), and the National Institutes of Health 1RO1-HL67110-01-A1 (Allan).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sayegh MH, Turka LA. The Role of T-Cell Costimulatory Activation Pathways in Transplant Rejection. N Eng J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 2.Lee RS, Yamada K, Houser SL, et al. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoji T, Wain JC, Houser SL, et al. Indirect recognition of MHC class I allopeptides accelerates lung allograft rejection in miniature swine. Am J Transplant. 2005;5:1626–1634. doi: 10.1111/j.1600-6143.2005.00925.x. [DOI] [PubMed] [Google Scholar]
- 4.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66–75. [PubMed] [Google Scholar]
- 5.Allan JS, Wain JC, Schwarze ML, et al. Modeling chronic lung allograft rejection in miniature swine. Transplantation. 2002;73:447–453. doi: 10.1097/00007890-200202150-00020. [DOI] [PubMed] [Google Scholar]


