Abstract
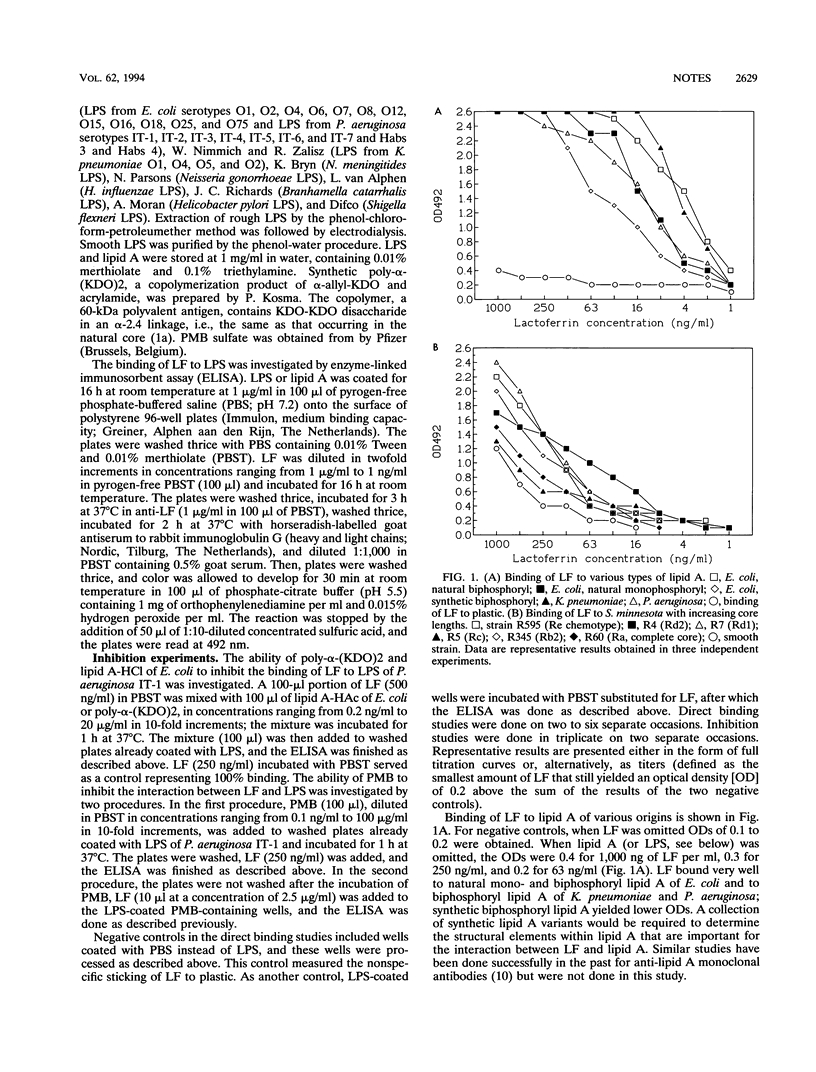
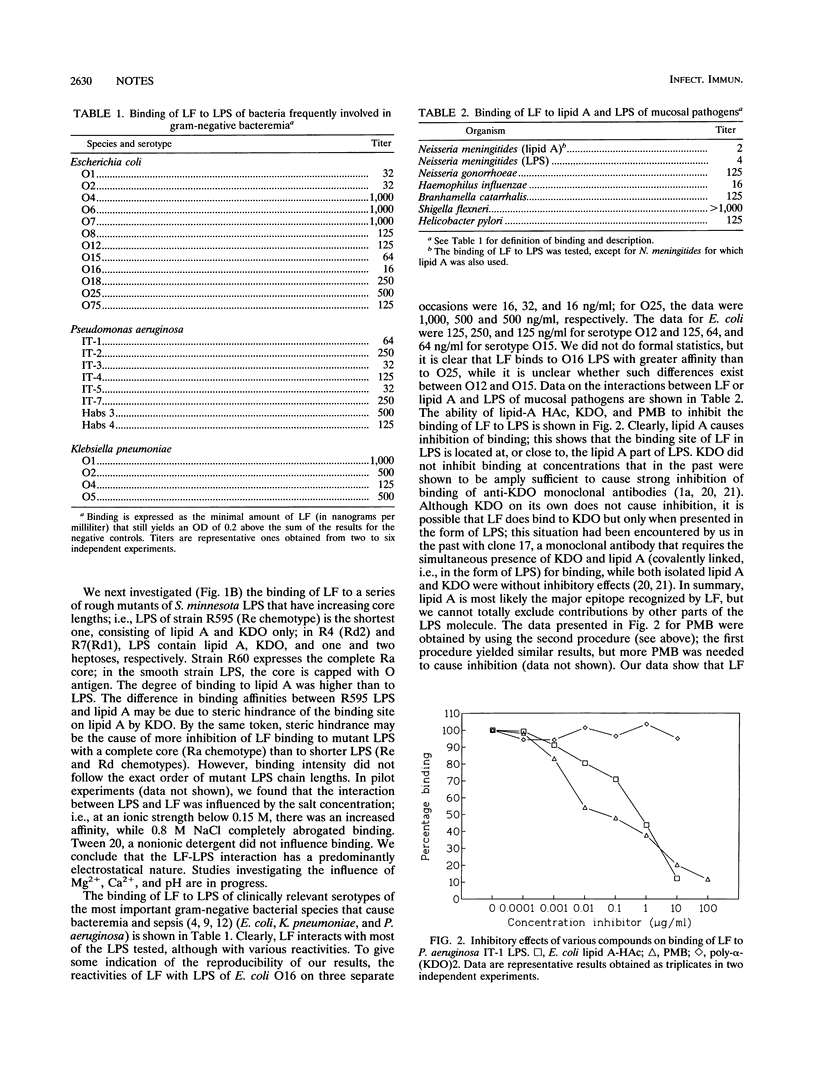
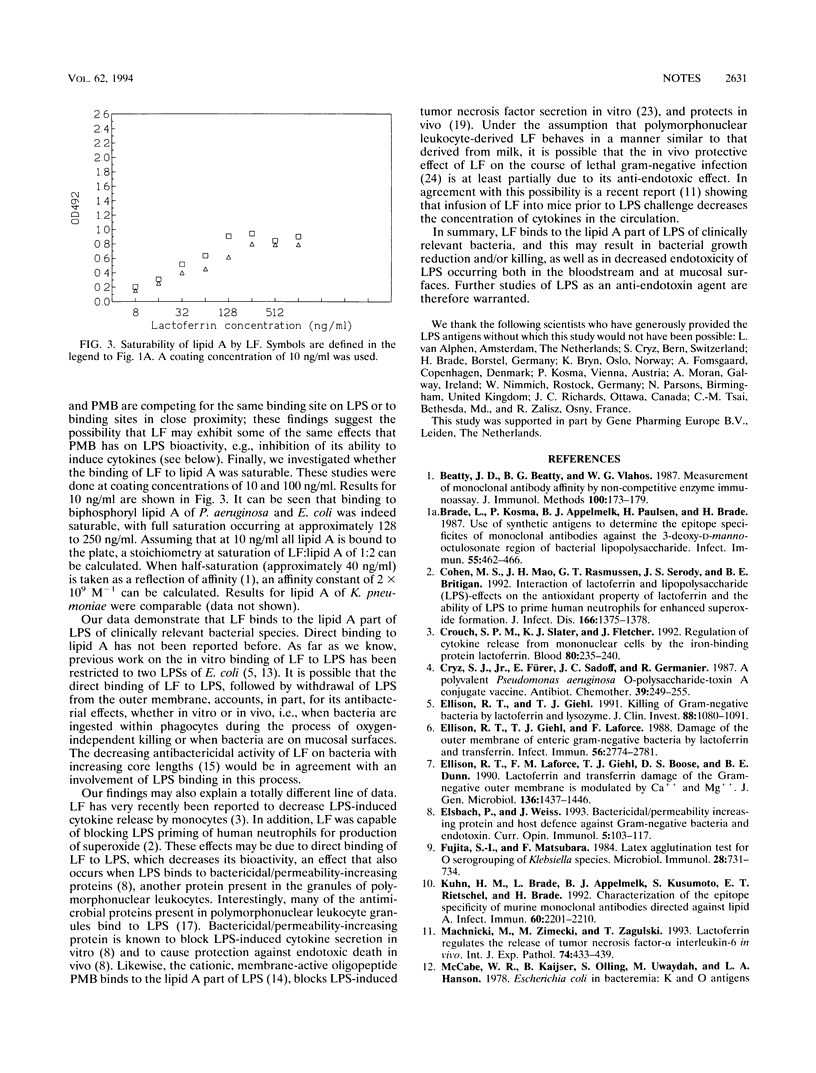
Lactoferrin (LF), a cationic 80-kDa protein present in polymorphonuclear leukocytes and in mucosal secretions, is known to have antibacterial effects on gram-negative bacteria, with a concomitant release of lipopolysaccharides (LPS, endotoxin). In addition, LF is known to decrease LPS-induced cytokine release by monocytes and LPS priming of polymorphonuclear leukocytes. Its mechanism of action is incompletely understood. We have now demonstrated by in vitro-binding studies that LF binds directly to isolated lipid A and intact LPS of clinically relevant serotypes of the species which most frequently cause bacteremia (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa), as well as to lipid A and LPS of mucosal pathogens (among others, Neisseria meningitides and Haemophilus influenzae). Binding to LPS was inhibitable by lipid A and polymyxin B but not by KDO (3-deoxy-D-manno-octulosonate), a glycoside residue present in the inner core of LPS. Binding of LF to lipid A was saturable, and an affinity constant of 2 x 10(9) M-1 was calculated for the LF-lipid A interaction. Our data may explain, in part, the mechanism whereby LF exerts its antibacterial and anti-endotoxic effects. Further studies on the ability of LF to block the detrimental effects of LPS, both in vitro and in vivo, are warranted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty J. D., Beatty B. G., Vlahos W. G. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987 Jun 26;100(1-2):173–179. doi: 10.1016/0022-1759(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Brade L., Kosma P., Appelmelk B. J., Paulsen H., Brade H. Use of synthetic antigens to determine the epitope specificities of monoclonal antibodies against the 3-deoxy-D-manno-octulosonate region of bacterial lipopolysaccharide. Infect Immun. 1987 Feb;55(2):462–466. doi: 10.1128/iai.55.2.462-466.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Mao J., Rasmussen G. T., Serody J. S., Britigan B. E. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J Infect Dis. 1992 Dec;166(6):1375–1378. doi: 10.1093/infdis/166.6.1375. [DOI] [PubMed] [Google Scholar]
- Crouch S. P., Slater K. J., Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992 Jul 1;80(1):235–240. [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Sadoff J. C., Germanier R. A polyvalent Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Antibiot Chemother (1971) 1987;39:249–255. doi: 10.1159/000414350. [DOI] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991 Oct;88(4):1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J., LaForce F. M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988 Nov;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, LaForce F. M., Giehl T. J., Boose D. S., Dunn B. E. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+. J Gen Microbiol. 1990 Jul;136(7):1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. Bactericidal/permeability increasing protein and host defense against gram-negative bacteria and endotoxin. Curr Opin Immunol. 1993 Feb;5(1):103–107. doi: 10.1016/0952-7915(93)90088-a. [DOI] [PubMed] [Google Scholar]
- Fujita S., Matsubara F. Latex agglutination text for O serogrouping of Klebsiella species. Microbiol Immunol. 1984;28(6):731–734. doi: 10.1111/j.1348-0421.1984.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Brade L., Appelmelk B. J., Kusumoto S., Rietschel E. T., Brade H. Characterization of the epitope specificity of murine monoclonal antibodies directed against lipid A. Infect Immun. 1992 Jun;60(6):2201–2210. doi: 10.1128/iai.60.6.2201-2210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicki M., Zimecki M., Zagulski T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int J Exp Pathol. 1993 Oct;74(5):433–439. [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K., Mantel C., Lu L., Morrison D. C., Broxmeyer H. E. Lactoferrin-lipopolysaccharide interactions. Effect on lactoferrin binding to monocyte/macrophage-differentiated HL-60 cells. J Immunol. 1991 Jan 15;146(2):723–729. [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Naidu S. S., Svensson U., Kishore A. R., Naidu A. S. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1993 Feb;37(2):240–245. doi: 10.1128/aac.37.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijens J. H., Abbink J. J., Wachtfogel Y. T., Colman R. W., Eerenberg A. J., Dors D., Kamp A. J., Strack van Schijndel R. J., Thijs L. G., Hack C. E. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992 Feb;119(2):159–168. [PubMed] [Google Scholar]
- Otto B. R., Verweij-van Vught A. M., MacLaren D. M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18(3):217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967 Apr;93(4):1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozalski A., Brade L., Kosma P., Appelmelk B. J., Krogmann C., Brade H. Epitope specificities of murine monoclonal and rabbit polyclonal antibodies against enterobacterial lipopolysaccharides of the Re chemotype. Infect Immun. 1989 Sep;57(9):2645–2652. doi: 10.1128/iai.57.9.2645-2652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozalski A., Brade L., Kuhn H. M., Brade H., Kosma P., Appelmelk B. J., Kusumoto S., Paulsen H. Determination of the epitope specificity of monoclonal antibodies against the inner core region of bacterial lipopolysaccharides by use of 3-deoxy-D-manno-octulosonate-containing synthetic antigens. Carbohydr Res. 1989 Oct 31;193:257–270. doi: 10.1016/0008-6215(89)85124-9. [DOI] [PubMed] [Google Scholar]
- Stokes D. C., Shenep J. L., Fishman M., Hildner W. K., Bysani G. K., Rufus K. Polymyxin B prevents lipopolysaccharide-induced release of tumor necrosis factor-alpha from alveolar macrophages. J Infect Dis. 1989 Jul;160(1):52–57. doi: 10.1093/infdis/160.1.52. [DOI] [PubMed] [Google Scholar]
- Sánchez L., Calvo M., Brock J. H. Biological role of lactoferrin. Arch Dis Child. 1992 May;67(5):657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagulski T., Lipiński P., Zagulska A., Broniek S., Jarzabek Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br J Exp Pathol. 1989 Dec;70(6):697–704. [PMC free article] [PubMed] [Google Scholar]



