Abstract
There is great interest in the structure of adiponectin as its oligomeric state may specify its biological activities. It occurs as a trimer, a hexamer and a high molecular weight complex. Epidemiological data indicates that the high molecular weight form is significant with low serum levels in type 2 diabetics but to date, has not been well-defined. To resolve this issue, characterization of this oligomer from bovine serum and 3T3-L1 adipocytes by sedimentation equilibrium centrifugation and gel electrophoresis respectively, was carried out, revealing that it is octadecameric. Further studies by dynamic light scattering and electron microscopy established that bovine and possibly mouse high molecular weight adiponectin is C1q-like in structure.
1. Introduction
Adiponectin is an adipocyte-derived hormone that has broad effects on whole body metabolism, from improving insulin sensitivity in the liver [1] to fatty acid oxidation in skeletal muscle [2], through the activation of AMP-activated protein kinase (AMPK)[3,4]. This adipokine is processed into at least three homomeric complexes, trimer, hexamer and “high molecular weight” (HMW) forms [5,6] and bears similarities in domain structure to the complement protein C1q [7]. There is growing interest in the role of these complexes in metabolism and disease as several lines of evidence suggest that oligmeric state affects the biological activity of adiponectin. At the molecular level, T-cadherin, an adiponectin receptor or co-receptor, shows selectivity in binding the hexamer and HMW forms, but not trimer [8]. Experiments using cell-based assays have also revealed differences in activity with oligomeric state, from activation of AMPK in skeletal muscle [9] to suppression of hepatic glucose output [6]. The “low molecular weight” form (probably a hexamer) reduces lipopolysaccharide-induced secretion of interleukin-6, which may form the basis for the anti-inflammatory properties of adiponectin [10]. Adiponectin has also been reported to mitigate endothelial cell apoptosis [11], and cell proliferation [12] in an oligomeric state-dependent manner. Physiologically, the sexual dimorphism with respect to complex distribution of adiponectin [6,13–17] has also been cited in connection with the possible significance of oligomeric state.
Recent studies are revealing that HMW adiponectin in particular is significant as it correlates well with various metabolic disorders. Type II diabetics have a low percentage of HMW adiponectin relative to total adiponectin (the SA index) compared to healthy individual [18]. Furthermore, thiazolidinediones, a class of antidiabetic drugs, improves this ratio. Follow-up work revealed that adiponectin knockout mice do not benefit from thiazolidinediones as do wild type mice [1]. These studies imply that the SA index may be a good marker for diabetes and that the antidiabetic action of thiazolidinediones involves HMW adiponectin. Furthermore, the SA index correlates tightly to glucose intolerance in non-diabetic Indo-Asian males [19], as well as with phenotypes associated with metabolic syndrome [20]. This trend continues in subjects who suffer from more advanced metabolic disorders [21]. These relationships are not surprising in light of the remarkable pharmacological properties exhibited by this protein [22], but the molecular basis by which HMW adiponectin exerts its effects are largely unknown. Most studies have focused on using the full-length protein, which is made up of multiple oligomers. To best study the mechanism of action however, as well as to advance its development as a therapeutic, it is necessary to work with discrete and well-characterized preparations of the HMW complex.
Accordingly, we set out to characterize this protein complex. Sedimentation equilibrium and electrophoretic studies of HMW adiponectin from bovine serum and culture of 3T3-L1 adipocytes show that it is octadecameric. Dynamic light scattering experiments and structural studies by transmission electron microscopy reveal that the HMW oligomer is structurally similar to C1q.
2. Materials and methods
2.1 Purification of Bovine HMW Adiponectin
HMW adiponectin was purified from calf serum (Hyclone) as described previously [23,24].
2.2 Sedimentation Equilibrium
Experiments were carried out using a Beckman XL-I analytical ultracentrifuge using 12 mm double sector Epon charcoal-filled centerpieces. HMW adiponectin (10 μM in monomer) in phosphate-buffered saline (PBS) pH=7.5 was centrifuged at 5000 rpm or 6000 rpm and 20°C for 18 hours prior to measurement of absorbance data. Data were fit to the following equation [25], that describes the gradient distribution of a homogeneous species: A = B + A′ exp[σ(x2−xo2)], where A = absorbance at radius x, A′ = absorbance at reference radius xo, σ = M (1−νρ)ω2/2RT, R = gas constant, T = temperature in Kelvin, ν = partial specific volume of adiponectin= 0.712 ml/g based on the partial specific volumes of its constituent amino acids (including hydroxyprolyl residues, see Supplementary Information), 4 glucosylgalactosyl [24], and 2 sialic acid moieties [23], ρ = density of solvent = 1.012 g/ml, ω= angular velocity in radians/s, M = apparent molecular weight, and B = solvent absorbance (blank).
2.3 Dynamic Light Scattering
Experiments were carried out in a Dynapro Titan dynamic light scattering instrument (Wyatt Technology Corporation). Data was acquired at 20°C, over 10s, repeated 10 times and averaged. Ten such acquisitions were performed to give 1000s of data. The Dynamics software was used to fit the autocorrelation function using the cumulants method, and to extract the diffusion coefficient and hydrodynamic radius. Particles smaller than 0.5 nm were excluded from the calculation of the percent mass as this signal also was present in the blank buffer.
2.4 Separation of HMW from Hexameric and Trimeric Adiponectin by Ion Exchange Chromatography
Murine HMW adiponectin from serum-free culture media of 3T3-L1 adipocytes was separated from the other oligomers by batch binding to DEAE Fast Flow Sepharose (Amersham) at 4°C, followed by elution with 25 mM Tris pH 8.0 containing 160 mM NaCl.
2.5 Thermal Unfolding of Murine HMW Adiponectin
The oligomer was heated with SDS loading buffer at the specified temperatures for 10 minutes prior to SDS-PAGE [15] on a 4–15% Tris-HCl gel (Bio-Rad) at 150 V. Visualization was achieved by western blot using a polyclonal antibody (R&D Systems).
2.6 Transmission Electron Microscopy of Bovine HMW Adiponectin
A 10 μM sample of bovine HMW adiponectin in PBS was diluted 9 fold with 50mM HEPES containing NaCl (150 mM) and CaCl2. The sample was applied to a glow-discharged carbon grid for 30s, blotted dry then stained with 1% uranyl acetate for 30s. Images were acquired using a Philips CM200 FEG transmission electron microscope (TEM) with a 2k by 2k Tietz CCD camera.
3. Results and discussion
3.1 Purification of bovine HMW adiponectin
Electrophoretic analysis of the purified bovine complex with visualization by silver staining (Figure 1A) revealed that it runs as a single band, indicating its high purity. Reduction followed by denaturation converted the slow running band to a small band moving at approximately 30 kDa (see Supplementary Information). The identities of both bands were verified to be adiponectin by western blot analysis.
Figure 1. Analysis of bovine HMW adiponectin by analytical ultracentrifugation.
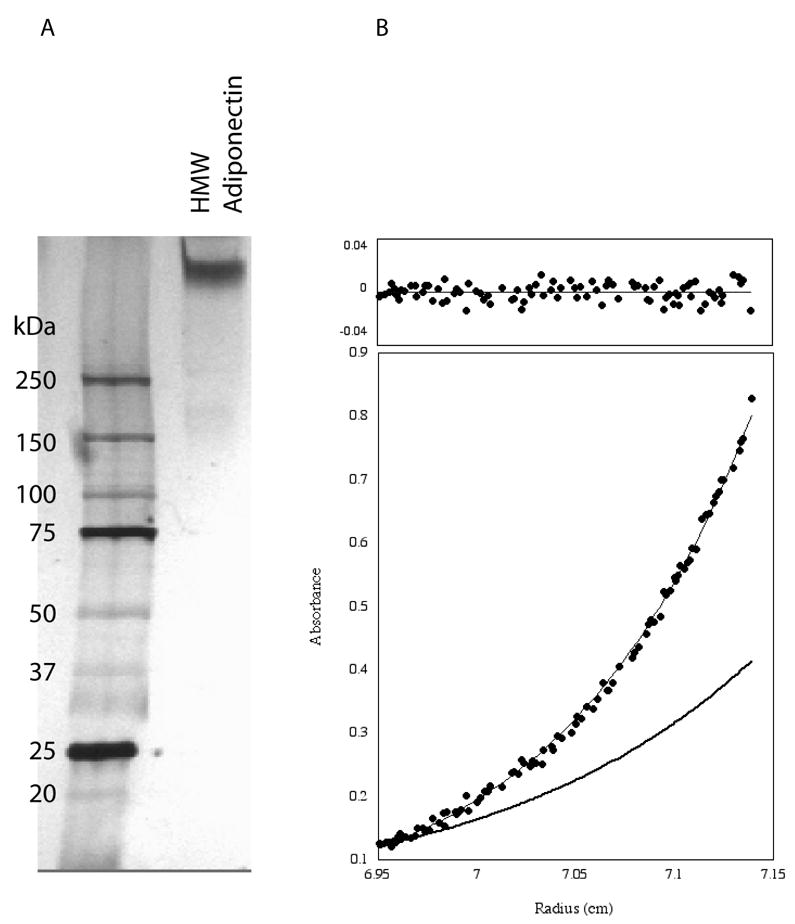
A. Electrophoretic analysis of purified bovine HMW adiponectin with silver staining. B. Sedimentation equilibrium trace of the purified complex. The data (circles) were fit using an ideal single species model and yielded an apparent molecular weight of 486 kDa. The residuals are shown above the trace. A theoretical curve (bold line) for a dodecamer is shown for comparison.
3.2 Bovine HMW adiponectin is an octadecamer
All sedimentation equilibrium experiments, carried out at 5000 rpm and 6000 rpm at 10 μM and 1 μM respectively, were consistent with HMW adiponectin being an octadecamer. Shown in Figure 1B is a typical trace obtained at 5000 rpm, along with residuals. All data sets fit best to an ideal single species model with random residuals (top). The random residuals provide further evidence that the sample is not undergoing measurable equilibrium with other oligomeric states. The apparent molecular weight, calculated from the average of 10 experiments, was determined to be 485 968 kDa, 18.4±1.1 times larger than the glycosylated monomer (26 455 Da). We conclude that HMW adiponectin is an octadecamer.
3.3 HMW adiponectin from 3T3-L1 adipocytes is an octadecamer
Figure 2A shows that murine HMW adiponectin has the same electrophoretic mobility as the bovine protein. This result suggests that it is also an octadecamer. The 2 smaller oligomeric states of murine adipoenctin were previously identified as hexamer and trimer by biophysical and imaging methods [5, 9]. Thus, knowing that the murine oligomers have 18, 6 and 3 subunits, a standard curve (shown in Figure 3C) was made from which the masses of intermediates generated by thermal denaturation at 90°C (Figure 2B) were estimated. In this lane, each intermediate band differed from the next by approximately 55 kDa (Figure 3C and Table 1), which is the mass of a dimer. Thus, it appears that thermal denaturation resulted in the generation of intermediates having 16, 14, 12, 10 or 8 subunits. A priori, this result is puzzling as the assumed minimal building block of HMW adiponectin is the trimer. The trimer however, is a non-covalent complex. Furthermore, disulfide bonds are required for HMW formation [6, 9, 15]. Since disulfide bonding is a covalent interaction, thermal denaturation would likely disrupt the trimer necessarily leading to loss of dimers. Presumably the third monomer remains disulfide bonded to the larger complex.
Figure 2. Murine adiponectin is an octadecamer.
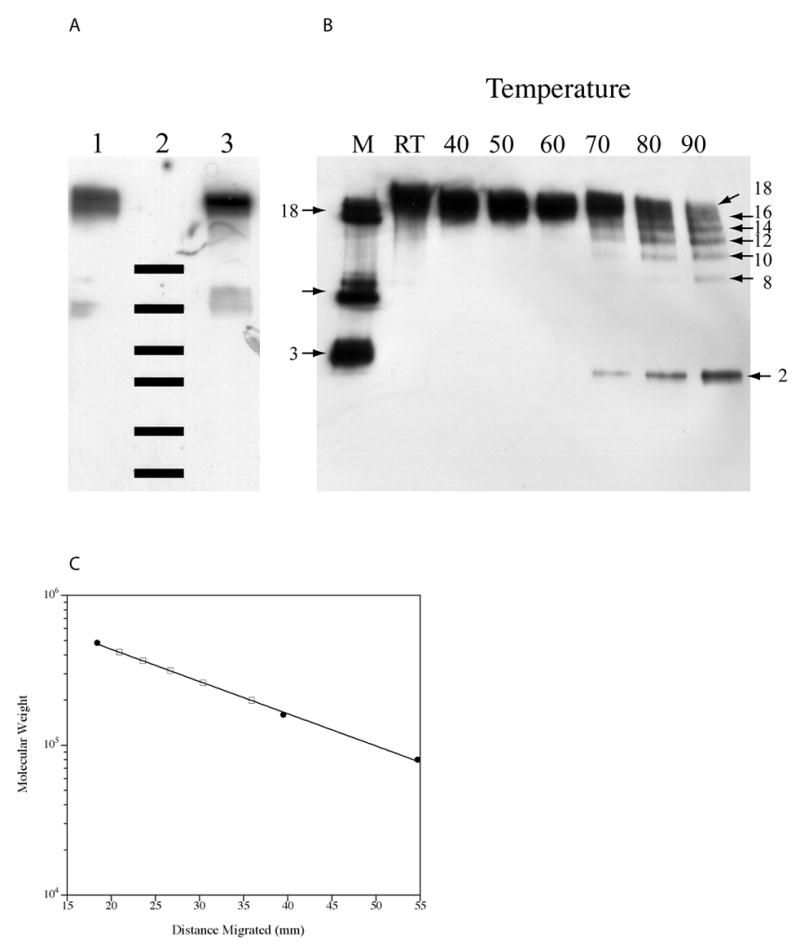
A. HMW adiponectin from 3T3-L1 adipocytes and from calf serum were analyzed by SDS-PAGE/western blot under non-reducing conditions. Lane 1: purified HMW complex from 3T3-L1 adipocytes; Lane 2: molecular weight markers, (250, 150, 100, 75, 50 and 37 kDa); Lane 3: bovine HMW adiponectin. B. Murine HMW adiponectin was heated to various temperatures for 10 minutes in non-reducing loading buffer prior to analysis by SDS-PAGE/western blot. The number of subunits in each oligomer are indicated. C. The molecular weights for the intermediates formed at 90°C (open squares) were interpolated from a standard curve obtained using the octadecamer, hexamer, and trimer (closed circles) from conditioned media of 3T3-L1 adipocytes.
Figure 3. Bovine HMW adiponectin has an apparent Stokes radius of 9 nm.
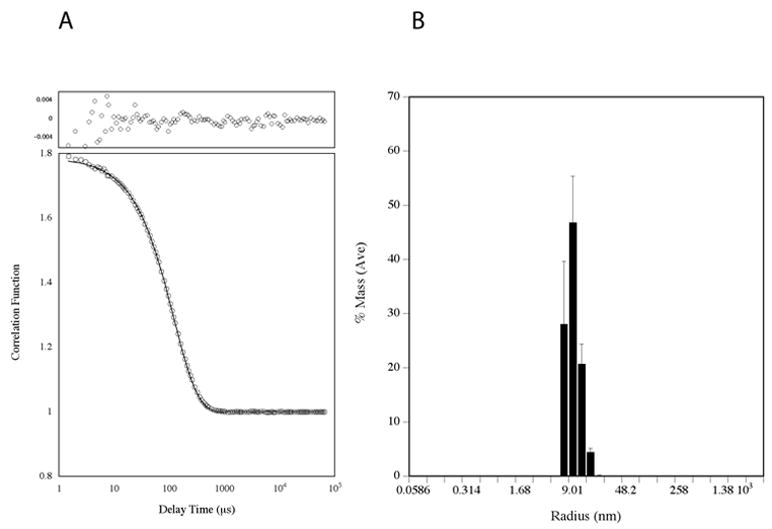
A. The autocorrelation function measured by dynamic light scattering. The data (circles) was fit by the method of cumulants (line). The residuals are plotted above. B. Size distribution of particles extracted from the autocorrelation function. Error bars represent standard deviations from 100 independent measurements.
Table 1.
Summary of the molecular weights of thermally denatured intermediates determined by gel electrophoresis
| Distance Migrated (mm) | Molecular Weight (kDa) | Number of Subunits |
|---|---|---|
| 20.9 | 419 | 16 |
| 23.6 | 366 | 14 |
| 26.7 | 314 | 12 |
| 30.4 | 261 | 10 |
| 35.9 | 199 | 8 |
3.4 HMW adiponectin is asymmetric in shape
Dynamic light scattering measurements gives additional data regarding the hydrodynamic size of the bovine complex. The autocorrelation function, shown in Figure 3A, is monoexponential as determined by the method of cumulants, [26] with random residuals, indicating that it is monodisperse and that there are no other physical processes that are occurring at detectable levels. The apparent hydrodynamic radius extracted from the autocorrelation function centers on 9.0 nm (Figure 3B). Using the calculated mass of octadecameric adiponectin, the theoretical size of an anhydrous 485 968 Da sphere is however estimated to be only 5.2 nm [27,28] (see Supplementary Information). Thus HMW adiponectin is asymmetric, which is expected if it were C1q-like in structure. From the Stoke-Einstein equation, the ratio of f/f o is 1.73. Modeled as an oblate ellipsoid, bovine HMW adiponectin has a maximal axial ratio = 11.8 with the long axes (2a) of the hydrated form being 26.8 nm in length and the short axis (2b) is 2.3 nm. The short axis seems unrealistically small but such an outcome was also obtained for C1q [29]. The long axes of the ellipsoid were comparable to the diameter of the splayed complexes observed by electron microscopy (2a=33 nm) but the short axis (2b) was a mere 2.2 nm in length. Thus, HMW adiponectin seems to have similar hydrodynamic behavior to C1q.
3.5 HMW adiponectin is structurally similar to C1q
The bouquet-like objects (Figure 4) observed for bovine HMW adiponectin by TEM provide additional insight into the structure of HMW adiponectin beyond prior EM studies [6, 9]. First, it is clearly reminiscent of C1q [30–32]. In flattened side views (bottom row), six globular objects can be seen atop thin stalks, which presumably correspond to the 6 trimers on collagen triple helices that are required to form the octadecameric complex. The stalks bunch together at the other end in a manner that is consistent with the requirement for N-terminal disulfide bonding [6,9,15]. While the disulifde-bond pattern of the complex remains to be elucidated, the overall architecture of HMW adiponectin is highly similar to C1q.
Figure 4. A montage of transmission electron micrographs of bovine HMW adiponectin.
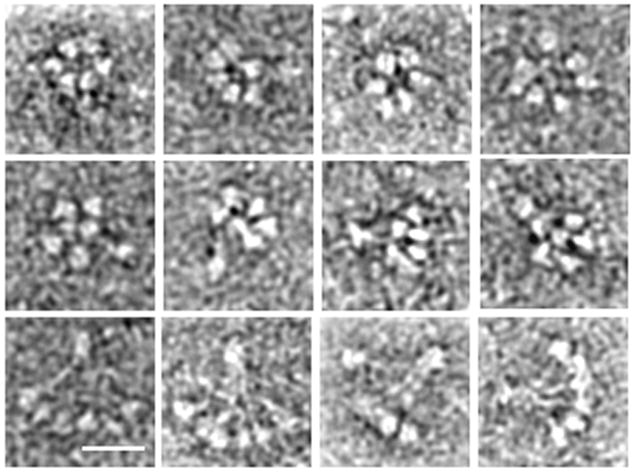
Top row: end views of HMW adiponectin with 6 globular heads arranged in a ring. Middle row: Side views of HMW adiponectin that highlight the conical shape of the complex. Bottom row: Side views of HMW adiponectin that appear flattened but highlight the similarity of the oligomer to C1q. Bar is 9.0 nm.
Some of the side views of HMW adiponectin (Figure 4, middle row) suggest a conical structure of the oligomer with the C-terminal portion forming the base. Interestingly, these globular domains are arranged in a tight ring. End-on views of the complex (Figure 4, top row) also show this arrangement, although some structures formed a circle of 5 globular domains plus one in the middle. The N-terminal region of the collagen domains sometimes appeared to be bundled together, implying higher order collagen interactions. Presumably, these interactions help direct the subunits to be parallel and form a closely packed cone,
The relevance of the oligomeric architecture of adiponectin to interactions with its receptor remains to be established. While this complex may possibly bind multiple receptors to bring about clustering, it is also plausible that the circular arrangement of the globular domains enable polyvalent interactions with a single receptor binding, perhaps in the middle. Such a mode of binding would not be available to the hexamer or trimer, and could therefore form the structural basis for differential signaling amongst adiponectin oligomers.
4. Conclusions
In summary, bovine and murine HMW complexes are octadecamers and the bovine adiponectin has a structure analogous to C1q. We are continuing studies to further our understanding of the relationship between oligomeric structure and biological activity and to facilitate the development of specific adiponectin oligomers as therapeutics to treat metabolic disorders.
Supplementary Material
Acknowledgments
We thank Uli Müller for training on the DLS instrument, and Ronald A. Milligan for access to EM facilities. We are grateful for financial support from the University of Arizona (T.-S. Tsao), NIH (1S10RR017948 to D.H. Lee; NIH-RO1-GM61938 to E.M. Wilson-Kubalek, and GM52468 to R.A. Milligan), NSF-(CHE-0320783 to D.H. Lee), Army Research Office (DAAD19-03-R-0005 to D.H. Lee), and Tufts University (to D.H. Lee). EM analysis was conducted at the National Resource for Automated Molecular Microscopy (NIH-P41 program, RR17573).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nawrocki AR, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–60. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 2.Fruebis J, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein. Nature Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 4.Tomas E, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protain kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 6.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 7.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 8.Hug C, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao TS, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–7. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 10.Neumeier M, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–8. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–7. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 13.Xu A, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 14.Gui Y, Silha JV, Murphy LJ. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res. 2004;12:1481–91. doi: 10.1038/oby.2004.185. [DOI] [PubMed] [Google Scholar]
- 15.Waki H, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 16.Hotta K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients Arterioscler. Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 17.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol. 2005;153:409–17. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 18.Pajvani UB, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 19.Fisher FF, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–7. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 20.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 21.Aso Y, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–60. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 22.Kadowaki T, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem. 2001;276:28849–56. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Proteomic and functional characterization of endogenous adiponectin purified from fetal bovine serum. Proteomics. 2004;4:3933–42. doi: 10.1002/pmic.200400826. [DOI] [PubMed] [Google Scholar]
- 25.Johnson ML, Correia JJ, Yphantis DA, Halvorson HR. Analysis of data from the analytical ultracentrifuge by nonlinear least-squares techniques. Biophys J. 1981;36:575–88. doi: 10.1016/S0006-3495(81)84753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisken BJ. Appl Optics. 2001;40:4087–4091. doi: 10.1364/ao.40.004087. [DOI] [PubMed] [Google Scholar]
- 27.Teller DC. Accessible area, packing volumes and interaction surfaces of globular proteins. Nature. 1976;260:729–31. doi: 10.1038/260729a0. [DOI] [PubMed] [Google Scholar]
- 28.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. In: Harding S, Rowe A, editors. Redwood Press Ltd; Melksham, UK: 1992. pp. 90–125. [Google Scholar]
- 29.Liberti PA, Paul SM. Gross conformation of C1q: a subcomponent of the first component of complement. Biochemistry. 1978;17:1952–8. doi: 10.1021/bi00603a023. [DOI] [PubMed] [Google Scholar]
- 30.Shelton E, Yonemasu K, Stroud RM. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq) Proc Natl Acad Sci U S A. 1972;69:65–8. doi: 10.1073/pnas.69.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slayter HS, Alexander RJ, Steiner LA. Electron microscopy of the complement protein C1q from the bullfrog, Rana catesbeiana. Eur J Immunol. 1983;13:102–6. doi: 10.1002/eji.1830130204. [DOI] [PubMed] [Google Scholar]
- 32.Brodsky-Doyle B, Leonard KR, Reid KB. Circular-dichroism and electron-microscopy studies of human subcomponent C1q before and after limited proteolysis by pepsin. Biochem J. 1976;159:279–86. doi: 10.1042/bj1590279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


