Abstract
Stability is one of the most fundamental concepts to characterize and evaluate any system. This term is often ambiguously used in spinal biomechanics. Confusion arises when the static analyses of stability are used to study dynamic systems such as the spine. Therefore, the purpose of this paper is to establish a common ground of understanding, using standard, well-defined terms to frame future discussions regarding spine dynamics, stability, and injury. A qualitative definition of stability, applicable to dynamic systems, is presented. Additional terms, such as robustness (which is often confused with stability) and performance are also defined. The importance of feedback control in maintaining stability is discussed. Finally, these concepts are applied to understand low back pain and risk of injury.
Keywords: spinal instability, lumbar spine, biomechanics, dynamics
1. The blind men and the elephant
John Godfrey Saxe's famous poem about six blind men and the elephant tells how different experiences lead to different perceptions. For the blind men, depending on what part was touched, they perceived the elephant to be a wall (side), spear (tusk), snake (trunk), tree (leg), fan (ear), or rope (tail). As the poem goes, these six blind men "disputed loud and long, each in his own opinion ~ exceeding stiff and strong, though each was partly in the right ~ and all were in the wrong!"
So how is this poem related to spine stability? The concept of "stability" has the potential to become our elephant. Stability, one could argue, is a term that appears to change depending upon the context, and as such, appears to have unstable definitions. The ambiguity of this term in spinal biomechanics should not be surprising, given that even in more established disciplines in engineering, there is no absolute definition of stability (Reeves and Cholewicki, 2003). However, numerous definitions have emerged, each rigorously defined. So like the elephant, stability is an entity with many parts. The key for the biomechanics community is to choose the appropriate definition that encapsulates the problems encountered with the spine. And since the spine is a dynamic system, it is important that the definition of stability reflects this attribute. Therefore, the goal of this paper is to establish a common ground of understanding, using standard, well-defined terms to frame future discussions regarding spine dynamics, stability, and injury - to avoid the pitfalls of the six blind men.
2. Why is stability important in spine biomechanics?
Stability is one of the most fundamental concepts to characterize and evaluate any system. For a system to carry out its goals or functions requires the system to be stable. In terms of the spine, stable behavior is critical for the spine to bear loads, allow movement, and at the same time avoid injury and pain.
Bergmark was the first to apply stability analysis to the spine by evaluating the potential energy of the system (Bergmark, 1987). (There are a number of simple explanations of static stability that interested readers can obtain (Crisco and Panjabi, 1990; McGill, 2001; McGill and Cholewicki, 2001)). This type of static analysis yielded a number of important insights including the requirement for stiffness from trunk muscles to maintain spine stability and prevent injury (Bergmark, 1989; Crisco and Panjabi, 1990; Crisco and Panjabi, 1991), the need for orchestrated recruitment (Bergmark, 1989), and the potential for injury under low level loading (Cholewicki and McGill, 1996). Concepts of "core stability" in rehabilitation emerged from these and other studies (Hides et al., 1994; Hides et al., 1996; Hodges and Richardson, 1996), and predictably, lack of stiffness was associated with injury. Prevention and rehabilitation efforts soon focused on retraining the central nervous system (CNS) to increase muscle recruitment in order to enhance spine stiffness to avoid low back pain (LBP). But is it always better to have a stiffer spine to reduce the risk of injury? Static definitions of spine stability would predict this to be the case.
In the past, a number of studies have documented an increase in force variability with increased muscle activation (Newell and Carlton, 1988; Sherwood et al., 1988; Carlton et al., 1993; Slifkin and Newell, 2000; Hamilton et al., 2004). If we consider the spine to be a dynamic system, then the importance of controller variability and its affects on stability needs to be addressed as well. Obviously, there are situations in which increased trunk stiffness is critical to protect the spine from injury (i.e., before a body check in hockey). But in situations where precise motor control is required (i.e., standing, balancing, or gait), is less activation and a more supple spine desirable? To address issues such as these requires dynamic characterization of stability.
Please note that because of limited space, only qualitative definitions of stability will be presented. However, future papers will describe quantitative methods for evaluating dynamic spine stability.
3. Stability, robustness, and performance
Although the focus of the paper is on stability, there are other system characteristics, such as robustness and performance, which are also important when describing a system's behavior. However, before assessing the robustness and performance of a system, the system must be stable. Consequently, we will start with the concept of stability.
3.1 Stability
To discuss stability of a system, whether it is in equilibrium (static) or changing with time (dynamic), we must give a small perturbation and observe the new behavior. If the new behavior is approximately the same as the old, qualitatively speaking, the system is stable. If the changed behavior becomes indistinguishable from the old behavior, returning to its original position or trajectory after a sufficiently long time, the system is asymptotically stable. Finally, if the disturbed behavior differs significantly from the old behavior, the system is unstable.
In terms of the spine, we are interested in the behavior of individual vertebrae and whether a perturbation to the system results in displacement beyond the physiological range. For instance, if a person catches an object thrown at them, a stable spine would deviate from its initial position slightly during the catch, but not enough to produce an injury. If the spine vertebrae return to their original position, the person's spine is asymptotically stable.
As stated earlier, stability has to be defined both for static conditions in which the system is in equilibrium, as well as for dynamic situations in which the system is moving along some trajectory. Regardless, the same concept applies. You apply a small perturbation and observe the new behavior. However, for the dynamic system, we are interested in comparing the new trajectory to the undisturbed trajectory. For instance, during a lift, the spine will follow an intended trajectory, representing a desired path/motion of the vertebrae. If during the lift, the load shifts or the person is bumped, a stable spine will have vertebrae that stay within the vicinity of or return to their intended trajectories.
"Everything should be made as simple as possible, but not simpler" (attributed to Einstein) is relevant to the above definition. For a precise definition of stability, further clarification is needed. For a system to be stable, it is necessary for the perturbed position to remain in the vicinity of the unperturbed position, but it is not sufficient. To clarify, not only does the system have to stay close to the initial position or trajectory, but it also needs to meet another requirement. This requirement evaluates whether we can limit the region in which the system lies. If you can limit the region in which the system lies by limiting the size of the perturbation, then the system is stable. If the region is the same size no matter what size of perturbation, then the system is unstable. This is an important distinction that needs to be understood.
If you tap someone's arm with a large force, you would expect the arm to deflect farther away than it would with a smaller tap. In this sense, we can limit the region in which the arm lies by controlling the perturbation size. Now consider a person with a neurological disorder that produces small oscillations. If we tap her/his arm, we may witness small oscillations around the original position. We could infer that since the new position remains in the vicinity of the original position, the arm is stable. But if we apply a smaller perturbation and the same size oscillations are produced, it would suggest that the region for the arm oscillations could not be limited. This system is unstable according to the revised definition.
3.1.1 Stability is context dependent
Stability depends on the system and the task being performed. This may also explain why there is so much confusion with the term. For instance, if one is investigating stability of standing, then a perturbation requiring a step could imply that stability was not maintained. But if the goal is to place a box on a shelf, if a perturbation required the person to take a step before the box is placed in its correct position, then it could be inferred that this task was successful and lifting was stable. Now if the goal is to lift the box without injuring the back, then not only does the lifter have to place the box in the correct place, but they must accomplish the task without generating injurious forces or excessive tissue strain.
3.2 Robustness
For practical reasons, we are interested in not only determining if a system is stable, but also how well it can cope with uncertainties and disturbances. For instance, sensory feedback is used to track the state of the spine, such as the position of vertebrae. Although, there is considerable information used to generate a neural representation of the spine system, it is still an approximation. If a controller is based on the approximate system, we hope that it would be capable of stabilizing the true system in spite of uncertainty regarding the system representation. Tolerance of such uncertainty is, qualitatively speaking, a problem of robustness.
We are also interested in the robustness of the system to disturbances. We hope that the system will be robust to perturbations and will maintain stable behavior for both small and large perturbations. In addition, systems that can significantly change their parameters (i.e. stiffness) without loss of stability are also said to be robust. We will discuss these concepts in more detail shortly.
3.2.1 A common mistake
One of the most common mistakes when characterizing a system is describing the level of stability of the system. This is an important point which we would like to highlight. A system is either stable or it is not - there should be no index or level of stability. Commonly, the term stability is often confused with robustness. To clarify, it is more appropriate to say the system is more robust than more stable. Core stabilizing exercises do not make the spine more stable, they make it more robust, thus reducing risk of injury.
3.3 Performance
Once the stability of a system is established, the interest shifts to its performance. Performance reflects how closely and rapidly the disturbed position of the system tends to the undisturbed position. Accuracy and speed are the two main attributes of any control system. Following a perturbation, a system performing well will have behavior that resembles the undisturbed behavior, suggesting that the error between disturbed and undisturbed motions is minimal. For asymptotically stable systems, a system performing well will also converge to the undisturbed position in a short time interval.
In most systems, there is a general relationship between performance and energy costs, with more energy required to improve performance. However, this is not always the case, particularly for the spine. There are situations in which increased energy (more muscle activation) does not result in improved performance (smaller vertebrae displacement). This will be discussed at the end of the paper in section 5.
3.4 The ball example
Many of the ideas discussed so far can be easily explained with a ball on a surface. The shape of the surface will indicate whether the ball is stable or unstable. In Figure 1a, the ball is unstable and will roll away. There is no size of perturbation small enough that will keep the ball close to the undisturbed position. Alternatively, stability can be assessed by a perturbation of the initial conditions. Moving the ball in Figure 1a, even slightly away, from the initial position will cause it to roll away. In Figure 1b, the ball is stable. The ball will be bounded, and with friction, will eventually return to its undisturbed position, making it asymptotically stable.
Figure 1a–b.
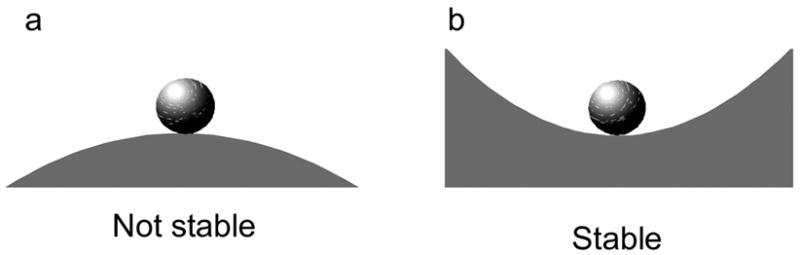
Stability of a ball.
The shape of the surface also determines how robust the system is to perturbations. In Figure 2a, the ball will have stable behavior for both small and large perturbations, whereas, the ball in Figure 2b will only be stable for small disturbances. Therefore, the first system is stable and robust. The second system is still stable but it is less robust.
Figure 2a–b.
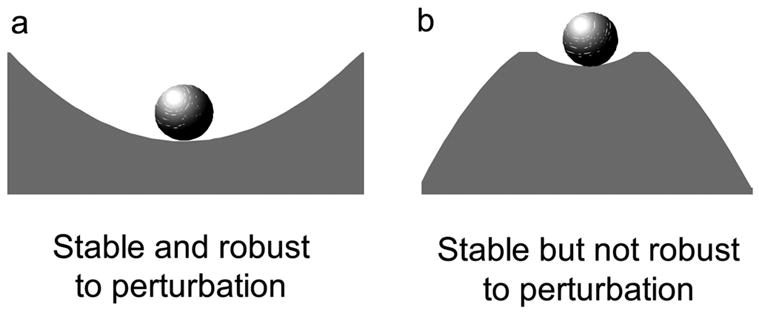
Robustness to perturbation.
As mentioned previously, robustness can also reflect a system's tolerance to changes in parameters. The steepness of the walls in the ball example reflects a parameter of the system. The sides in Figure 3a are steeper than 3b and can undergo more of a change in steepness before the system becomes unstable. The system in Figure 3b can only tolerate small changes in steepness suggesting it is less robust than the system in Figure 3a. This definition of robustness probably better reflects terms used in the biomechanics community. For instance, trunk stiffness reflects the steepness of the walls. Increased stiffness represents steeper sides indicating the spine is more robust than at lower stiffness levels. In a static spine system, individuals with higher levels of activation have a larger "margin of safety" than those with less activation (Cholewicki and McGill, 1996). However, it is important to point out that increased activation does not always produce a more robust spine as will be shown later when discussing dynamic spine stability (Section 5).
Figure 3a–b.
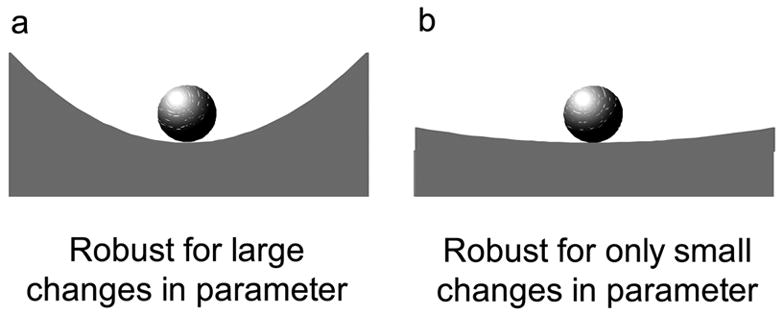
Robustness to change in system’s parameters.
Unless the system is stable, there is no reason to discuss its performance. All unstable systems perform poorly. For a stable system, the steepness of the walls will determine how well the system performs. Steeper walls, such as in Figure 3a, will keep the ball closer to the original undisturbed position and produce a faster response (faster movement of the ball).
4. Feedback control and stability
Given that the spine has similar characteristics to an inverted pendulum, it can be shown to be unstable and hence we assume that it will not behave "well". Therefore, we want to control it in some fashion (1) to ensure that it is stable, and (2) to improve its robustness and performance. The principal approach for control is feedback. The information concerning the output of the system is fedback and used to modify the input (Figure 4). In control parlance, the isolated system is called the plant, and in this case, represents the spine. The logic by which the control input is generated from the output is the controller, and in this case, a feedback controller. The plant (spine) together with the controller (feedback control) is the overall system (spine system). Simple and complex examples of feedback control systems are shown in Figures 4a and 4b respectively.
Figure 4a–b.
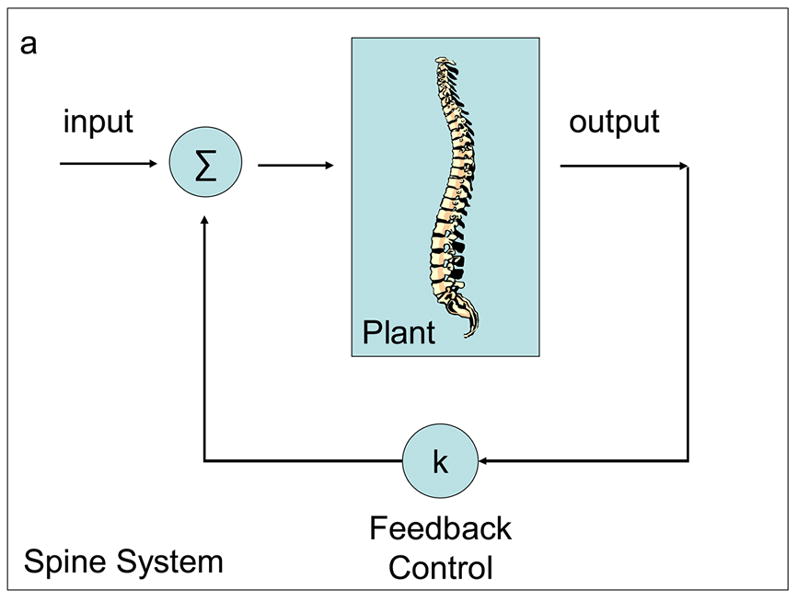
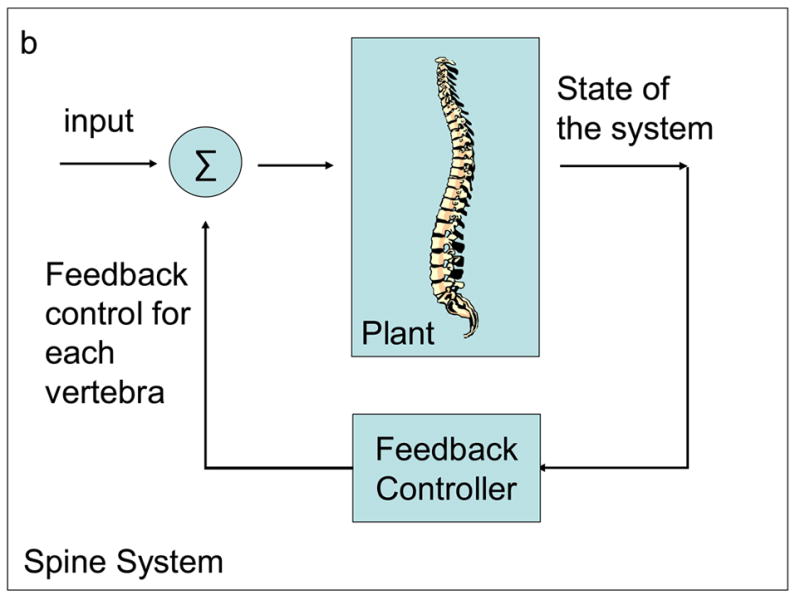
Feedback control of a spine.
In Figure 4a, the control input to the plant is proportional to its output. This is indicated by the feedback gain denoted by k. Feedback can be positive or negative. If positive, the system is unstable since the force is in the same direction as the displacement. For stability, negative feedback is used so that the force is generated in the direction to counteract the displacement. For the plant to return to the undisturbed position or trajectory, the feedback must tend to zero when the error between disturbed and undisturbed systems approaches zero. It is clear that this is achieved using negative feedback as shown in Figure 4a.
Figure 4a shows the simplest form of feedback control. In reality, the spine system is significantly more complex. As shown in Figure 4b, the feedback signals come from sensors that convey information about the state of the entire system. For instance, spinal position and velocity are likely monitored by muscle spindles (Buxton and Peck, 1989; Nitz and Peck, 1986) and other various mechanoreceptors embedded in the spinal tissue (Jiang et al., 1995; McLain, 1994; Mendel et al., 1992; Roberts et al., 1995). These signals are processed by the feedback controller, which in turn, generates several control signals to be applied at the different segmental levels.
For a static condition, where the spine is maintaining an upright posture, the input to the system will be zero. This means that the forces acting on the system are balanced producing zero net force. Consequently, if the spine is perturbed, the force applied to the system will only come from feedback control, which helps restore the system to the equilibrium position (Figure 5a). For a dynamic condition, where the spine is moving through some trajectory, the input into the system will not be zero, but instead will represent the muscle forces needed to produce the desired motion. If the spine is bumped during the movement, muscle forces generated from feedback control will be added to the input forces, and in this case, the feedback control will help restore the system’s movement to its intended trajectory (Figure 5b).
Figure 5a–b.
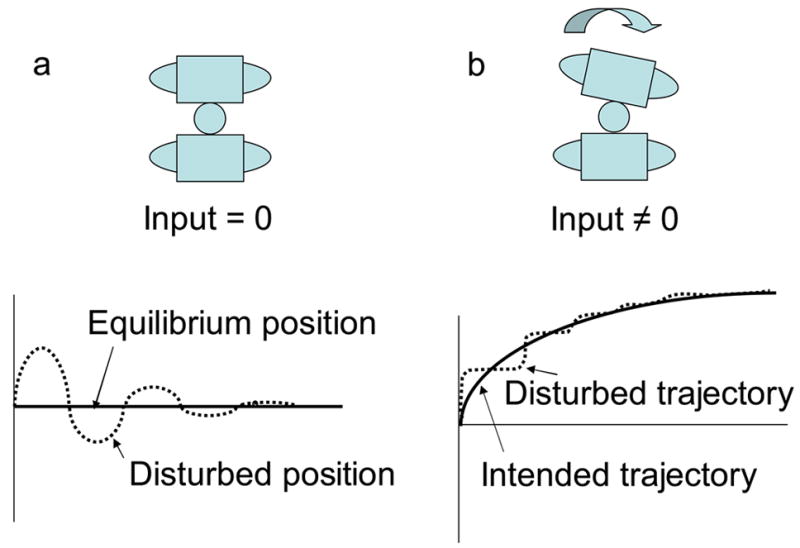
Response to perturbations in the spine.
4.1 Feedback control pathways of the spine
The feedback controller for the spine consists of intrinsic properties of intervertebral joints (i.e. joint stiffness and damping), intrinsic properties of trunk muscles (i.e. short range muscle stiffness and damping), and the CNS, which can respond to perturbations with both reflexive and voluntary muscle activation (Figure 6). Feedback control from intrinsic pathways is instantaneous, whereas feedback control from reflexive and voluntary pathways has inherent delays (Figure 6). These delays represent the time taken to sense a perturbation and respond with increased muscle activation to counteract the disturbance. Delays reflect signal transmission, CNS processing time, and time required to generate muscle force (electromechanical delay).
Figure 6.
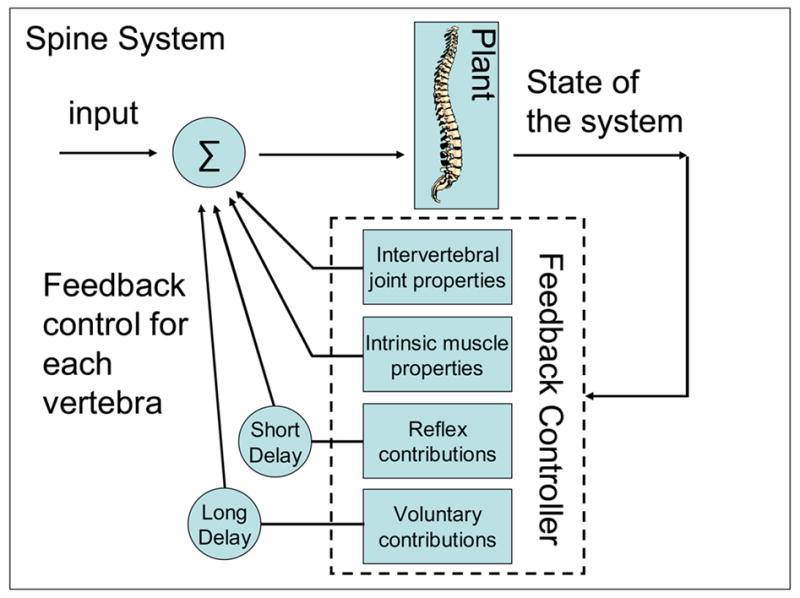
Components of the spine feedback controller.
In the presence of a disturbance, some or all of these feedback pathways can be utilized to provide the necessary and appropriate force to stabilize the spine. The contribution of each component changes depending on the size of the perturbation, as well as on the initial state of the spine system. For instance, feedforward input from the CNS can be used to increase trunk muscle co-activation prior to a perturbation, thus increasing muscle stiffness (and intervertebral stiffness as well (Stokes et al., 2002)) allowing the intrinsic properties of the system to contribute more to the perturbation than responses with delays. Indeed, several studies have shown that voluntarily pre-tensioning of trunk muscles obviates the need for a reflex response, which is less likely to occur following a perturbation (Stokes et al., 2000; Granata et al., 2004a). This strategy may be desirable in situations where the perturbation force is large and expected (i.e. a body check in hockey), since delays may cause stabilizing forces to act too late. Alternatively, in tasks that must be maintained for longer periods and require precise control (i.e. standing), relying on reflexive pathways might be a better choice, since it is metabolically more efficient than a muscle co-activation strategy. Because of the various feedback pathways, there is considerable flexibility in how the spine system can maintain stability. However, for each task, there is most likely an optimal control strategy that minimizes metabolic costs and/or maximizes the system’s performance.
Please note that further clarification regarding Figure 6 is necessary. The delineation between the plant and the controller is somewhat arbitrary. For instance, the intrinsic properties of the intervertebral joint and muscles could be included in the plant. We decided to include it as part of the controller to emphasize the fact that these two elements also provide feedback control to the spine, which is based on the state of the spine. For the remainder of this paper, we will assume that only the reflexive and voluntary pathways that travel through the spinal cord represent the feedback controller. The properties of the intervertebral joint and intrinsic properties of the trunk muscles will be included in the plant. With this new delineation, the logical connection between the feedback controller and the neural circuitry within the CNS can be made.
5. Plant versus system characteristics
In Figure 3a–b, a perturbation produces a deviation away from the original position or trajectory. A stiffer system will be displaced less than a compliant system and will respond faster, suggesting that it performs better. So it would appear that increasing spine stiffness through trunk muscle co-activation would (1) ensure that the spine is stable, (2) reduce the amount of displacement, and (3) minimize the risk of injury. However, one has to remember that stability is context dependent (Section 3.1.1). Indeed, a stiffer system will always perform better, but this statement refers to the entire system – plant together with its controller. On the other hand, a stiffer spine (plant) achieved through muscle co-activation does not include the remainder of reflexive and voluntary responses (controller). Thus, increased spine stiffness by itself does not always lead to better system performance. To demonstrate when too much trunk stiffness can be detrimental, we asked subjects to balance on an unstable surface (Reeves et al., 2006). This balancing task requires precise feedback control to maintain equilibrium. Subjects balanced with normal levels of trunk muscle activation and also with higher levels, reflecting two levels of spine (plant) stiffness. Obviously, some level of stiffness was required to maintain balance. But too much stiffness made the task more challenging and in some cases resulted in a loss of balance – an unstable system!
The purpose of the study was to show that increasing stiffness in one part of a system does not always increase the overall system's stiffness. Interestingly, we found that a stiffer spine resulted in more trunk displacement during the balancing task. In this case, the "balancing system" is less stiff when the spine stiffness (plant) exceeded some optimal level. As mentioned already, muscle force variability increases as muscle activation increases. Because the CNS is trying to maintain balance, the spine undergoes larger excursions when the feedback controller is less precise. This variability in the feedback controller results in the overall system being less stiff. The next statement is important in the context of injury risk. Because the spine is stiffer, more muscle force is required to produce the displacement to "catch" ones balance. An undesirable situation arises where the spine undergoes more displacement while under greater load. Consequently, for some activities requiring precise trunk control, risk of injury could increase with having a trunk that is too stiff! This would not have been predicted with static characterization of stability.
The seated balance task illustrates that the trade-off between performance and energy does not always hold for biological systems. Increased trunk muscle activation does not always yield less displacement following a perturbation. Internal disturbances can also act to destabilize the system. For tasks requiring precise motor control, such as a logger walking across a fallen tree, the goal should be to find the optimal level of trunk stiffness to ensure the task can be successfully completed while at the same time minimizing risk of injury.
6. Feedback control model of LBP
Now that the framework for discussing the spine system has been presented, we can apply it to better understand biomechanical aspects of LBP. Clearly, there are many pathways for the development of LBP. In this section, we will discuss a number of possible mechanisms by which impairment in feedback control can lead to pain and injury. This is not intended to be an exhaustive list, but rather a few examples of how the presented framework encompasses various scenarios. To start, we will review the orthopaedic notion of spine instability to show how it fits a model of injury based on impaired feedback control.
6.1 Plant related impairment
In the past, lack of or reduced intervertebral stiffness was associated with clinical spinal instability (Panjabi, 1992). In the true sense of the definition, it may not be instability, but regardless, altered passive stiffness may lead to LBP. As was mentioned already, displacement of the vertebra results in a counteracting force generated by the passive tissue that is proportional to the size of displacement. Lack of or reduced stiffness in any degree-of-freedom of the spine means that the counteracting force used to stabilize the spine is reduced. This could be interpreted as impaired feedback control.
It is possible that the CNS observing a lack of interverbral stiffness could compensate by increasing trunk muscle activation to maintain spine stability (Figure 7). Modeling of injury has shown that a reduction of intervertebral stiffness could be compensated by a small increase of 1–2 % maximum voluntary activation (% MVA) in trunk muscle co-activation (Cholewicki et al., 1997). Interestingly, several studies have shown that LBP patients tend to have higher levels of co-activation of trunk muscles than healthy controls (Marras et al., 2001; Lariviere et al., 2002; Van Dieen et al., 2003). This small increase in muscle activation, although slight, might have clinical significance.
Figure 7.
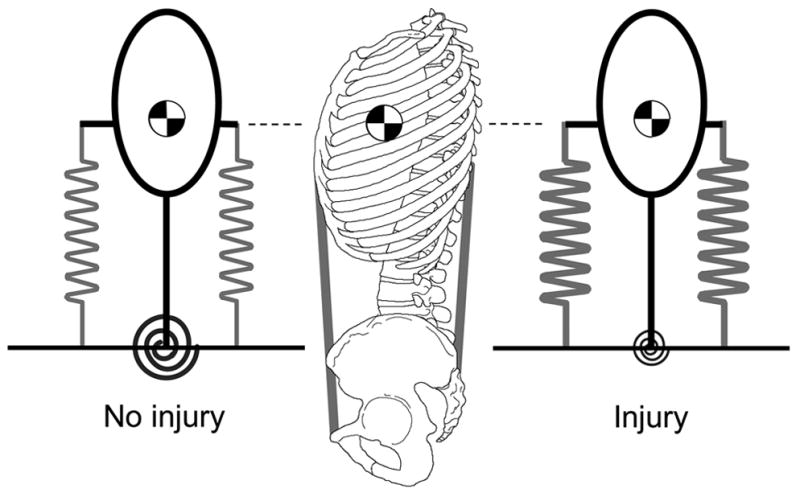
The CNS can compensate for a reduction in intervertebral stiffness by increasing trunk muscle co-activation. The passive stiffness of the intervertebral joint is represented by the torsional spring at the base of the inverted pendulum.
Static muscular contractions sustained over long periods, even at low levels, can lead to fatigue and the manifestation of pain (Jonsson, 1978). But there appears to be a threshold at 5% MVA, below which muscle contraction can be sustained indefinitely (Bjorksten and Jonsson, 1977). Both modeling and experimental results have shown that healthy controls operate below this critical 5 % MVA threshold for most postural activities like standing, walking, sitting that must be maintained throughout the day (Cholewicki et al., 1997). So it is plausible that even a slight increase in muscle activation following injury could elevate muscle contraction above the critical threshold, resulting in fatigue-related pain. Shortly, we will discuss the effects of fatigue on the feedback controller.
6.2 Controller related impairment
Feedback control can stabilize the system if and only if the controller can obtain information regarding the state of the system and has sufficient force generating capacity to control all degrees of freedom in a desired fashion. In terms of the spine, (1) sufficient sensory information is needed to track the position of all the vertebrae (observability), and (2) sufficient muscular attachments to the spine are necessary to move each vertebra along a desired path (controllability). If either of these two conditions is not met, the system may become unstable.
Observability and controllability are not really an issue in the spine since the spine has an over-redundancy in sensory representation and muscle force generation. The issues are more related to accuracy of sensory information, precision by which muscles can be controlled, and their capacity to generate force. If noise enters the system from either the sensory side or the muscle force generation side, the performance of the system will suffer, and in the extreme case, the spine could become unstable. Similarly, limited force generating capacity of muscles (e.g. weakness or fatigue) can also lead to spine instability if high muscle forces are required to stabilize the system.
Obviously, fatigue can limit muscle force generating capacity (Burke et al., 1973). But fatigue also poses other more subtle dilemmas for the spine system. Fatigue has been shown to impair spine proprioception (Taimela et al., 1999) and the ability to regulate force (Parnianpour et al., 1988; Sparto et al., 1997). Recently, we conducted an experiment that showed trunk muscle force variability increases after a fatiguing trial, and like others found that fatigue also produces more trunk muscle co-activation (O'Brien and Potvin, 1997; Potvin and O'Brien, 1998; Granata et al., 2004b). Perhaps this co-activation strategy reflects the CNS attempt to maintain the force generating capacity of the trunk muscles (Herrmann et al., 2006) or possibly reflects a compensatory strategy for impaired feedback control. Unfortunately, as pointed out already, this strategy is metabolically costly and could impair feedback control even further, resulting in a vicious cycle. Perhaps, LBP patients with less fatigue-resistant muscle fibers (Mannion et al., 1997) are trapped in this vicious cycle, which could explain their tendency to exhibit more trunk co-activation.
Clearly, there are a host of possible risk factors that can lead to impaired feedback control such as prolong flexion (Solomonow et al., 2003; Granata et al., 2005; Rogers and Granata, 2006), whole body vibration (Roll et al., 1980; Gauthier et al., 1981); and muscle wasting (Hides et al., 1996) to name a few. Delayed reflex responses are another source for controller impairment that has been associated with LBP (Radebold et al., 2000; Radebold et al., 2001; Reeves et al., 2005), and more recently has been shown to increase the risk of injury (Cholewicki et al., 2005). Delays in feedback control act to destabilize the system. In environments with sudden and unexpected loads, delayed responses may result in feedback control that is too late or inappropriate, making the spine system more prone to injury.
6.3 Effects of injury on feedback control strategies
What happens to feedback control immediately following an injury? There are some possible sensory changes following injury (i.e. loss of sensory mechanoreceptors, pain) that could affect the input to the controller. Moreover, tissue damage or deformation (i.e. swelling) in the oesteoligamentous spine, if significant, could alter its mechanical properties and the "logic" for controlling the plant (spine) may no longer be valid. Discrepancies between injured and uninjured spine mechanics and a loss or disruption of sensory signals regarding the system’s state may compromise the feedback controller's ability to maintain spine stability. Perhaps sensing this, the CNS compensates by "locking-up" the back through massive trunk muscle co-activation, indicative of muscle spasm. There is some evidence to suggest that for highly unstable environment and/or novel tasks the CNS uses muscle co-activation to maintain joint stability (Milner and Cloutier, 1993; Franklin et al., 2003; Milner, 2002).
6.4 Spinal instability: Myth or reality?
Although, spinal instability is a commonly cited cause of injury and pain, it is unclear if LBP results from unstable behavior of the spine. As has been suggested in previous sections, a spine system that does not behave "well" is more prone to injury, but does spine instability actually occur? Preuss and Fung (2005) provide a nice review on the potential links between LBP and spinal instability. They highlight a number of experimental and modeling studies that suggest an error in neuromuscular control could result in unstable segmental behavior. During a loss of spinal stability, damage to the osteoligmentous spine could occur if a vertebra displacement exceeds physiological limits. Such an injury event was witnessed in a powerlifter during a deadlift (Cholewicki and McGill, 1992). Alternatively, they suggested that over-activation of important stabilizing segmental muscles, following a transient period of instability could produce an injury if strain in these small muscles exceeds their capacity. This could explain the sudden onset of LBP during benign activities such as picking-up a newspaper.
It is also important to consider that a painful muscle spasm could occur without injury or loss of spine stability. It is conceivable that this “locking-up” response could be invoked when the controller senses the spine being on the verge of instability. This hypothesis originates from the studies demonstrating that a real or perceived threat of injury or pain leads to significant changes in trunk muscle recruitment pattern (Hodges et al., 2003; Moseley et al., 2004).
Clearly, more work is needed to investigate these and other potential injury mechanisms to see if the link exists between instability and LBP. But we know for certain that the spine must be stable to function properly and to fulfill its physiological role.
7. Conclusions
Stability is an elusive term that could lead to confusion. To help facilitate future discussions, a formal definition and a framework was presented which encompasses both static and dynamics spine systems. When considering the spine as a dynamic system, a static concept of spine stability is not adequate for discussing the involved issues. The stability of the system is maintained with feedback control, which also affects the system’s robustness and performance.
Stability is context dependent; therefore, it is important to frame the definition of stability in terms of the task and the system. Care must be taken not to confuse spine (plant) characteristics with the overall system’s characteristics. A stiffer spine does not always imply a stiffer overall system. In some cases, a stiffer spine may experience larger displacements to maintain task stability, which can increase the risk of injury.
Impairment of feedback control has implications for LBP. For example, tissue damage, degradation of sensory information, muscle force variability all affect the spine system's performance, robustness, and potentially stability. If the system does not perform well, it is more prone to injury.
Acknowledgments
This publication was made possible by the NIH Grant Number R01 AR051497 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergmark A. Mechanical Stability of the Human Lumbar Spine. Department of Solid Mechanics; Lund University: 1987. [Google Scholar]
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Bjorksten M, Jonsson B. Endurance limit of force in long-term intermittent static contractions. Scand J Work Environ Health. 1977;3:23–7. doi: 10.5271/sjweh.2795. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–48. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton DF, Peck D. Neuromuscular spindles relative to joint complexities. Clinical anatomy. 1989;2:211–24. [Google Scholar]
- Carlton LG, Kim KH, Liu YT, Newell KM. Impulse variability in isometric tasks. J Mot Behav. 1993;25:33–43. doi: 10.1080/00222895.1993.9941637. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Lumbar posterior ligament involvement during extremely heavy lifts estimated from fluoroscopic measurements. J Biomech. 1992;25:17–28. doi: 10.1016/0021-9290(92)90242-s. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Panjabi MM, Khachatryan A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine. 1997;22:2207–12. doi: 10.1097/00007632-199710010-00003. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Silfies SP, Shah RA, Greene HS, Reeves NP, Alvi K, Goldberg B. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine. 2005;30:2614–20. doi: 10.1097/01.brs.0000188273.27463.bc. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Panjabi MM. Postural Biomechanical Stability and Gross Muscular Architecture in the Spine. In: Winters JM, Woo SL, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. New York: Springer-Verlag; 1990. pp. 438–450. [Google Scholar]
- Crisco JJ, 3rd, Panjabi MM. The intersegmental and multisegmental muscles of the lumbar spine. A biomechanical model comparing lateral stabilizing potential. Spine. 1991;16:793–9. doi: 10.1097/00007632-199107000-00018. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Osu R, Burdet E, Kawato M, Milner TE. Adaptation to stable and unstable dynamics achieved by combined impedance control and inverse dynamics model. J Neurophysiol. 2003;90:3270–82. doi: 10.1152/jn.01112.2002. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Roll JP, Martin B, Harlay F. Effects of whole-body vibrations on sensory motor system performance in man. Aviat Space Environ Med. 1981;52:473–9. [PubMed] [Google Scholar]
- Granata KP, Rogers E, Moorhouse K. Effects of static flexion-relaxation on paraspinal reflex behavior. Clin Biomech. 2005;20:16–24. doi: 10.1016/j.clinbiomech.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata KP, Slota GP, Bennett BC. Paraspinal muscle reflex dynamics. J Biomech. 2004a;37:241–7. doi: 10.1016/s0021-9290(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Granata KP, Slota GP, Wilson SE. Influence of fatigue in neuromuscular control of spinal stability. Hum Factors. 2004b;46:81–91. doi: 10.1518/hfes.46.1.81.30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Jones KE, Wolpert DM. The scaling of motor noise with muscle strength and motor unit number in humans. Exp Brain Res. 2004;157:417–30. doi: 10.1007/s00221-004-1856-7. [DOI] [PubMed] [Google Scholar]
- Herrmann CM, Madigan ML, Davidson BS, Granata KP. Effect of lumbar extensor fatigue on paraspinal muscle reflexes. J Electromyogr Kinesiol. 2006;16:637–641. doi: 10.1016/j.jelekin.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–9. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–72. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res. 2003;151:262–71. doi: 10.1007/s00221-003-1457-x. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–50. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Jiang H, Russell G, Raso VJ, Moreau MJ, Hill DL, Bagnall KM. The nature and distribution of the innervation of human supraspinal and interspinal ligaments. Spine. 1995;20:869–76. doi: 10.1097/00007632-199504150-00001. [DOI] [PubMed] [Google Scholar]
- Jonsson B. Quantitative electromyographic evaluation of muscular load during work. Scand J Rehabil Med Suppl. 1978;6:69–74. [PubMed] [Google Scholar]
- Lariviere C, Gagnon D, Loisel P. A biomechanical comparison of lifting techniques between subjects with and without chronic low back pain during freestyle lifting and lowering tasks. Clin Biomech. 2002;17:89–98. doi: 10.1016/s0268-0033(01)00106-1. [DOI] [PubMed] [Google Scholar]
- Mannion AF, Weber BR, Dvorak J, Grob D, Muntener M. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Orthop Res. 1997;15:881–7. doi: 10.1002/jor.1100150614. [DOI] [PubMed] [Google Scholar]
- Marras WS, Davis KG, Maronitis AB. A non-MVC EMG normalization technique for the trunk musculature: Part 2. Validation and use to predict spinal loads. J Electromyogr Kinesiol. 2001;11:11–8. doi: 10.1016/s1050-6411(00)00040-7. [DOI] [PubMed] [Google Scholar]
- McGill SM. Low back stability: from formal description to issues for performance and rehabilitation. Exerc Sport Sci Rev. 2001;29:26–31. doi: 10.1097/00003677-200101000-00006. [DOI] [PubMed] [Google Scholar]
- McGill SM, Cholewicki J. Biomechanical basis for stability: an explanation to enhance clinical utility. J Orthop Sports Phys Ther. 2001;31:96–100. doi: 10.2519/jospt.2001.31.2.96. [DOI] [PubMed] [Google Scholar]
- McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19:495–501. doi: 10.1097/00007632-199403000-00001. [DOI] [PubMed] [Google Scholar]
- Mendel T, Wink CS, Zimny ML. Neural elements in human cervical intervertebral discs. Spine. 1992;17:132–5. doi: 10.1097/00007632-199202000-00002. [DOI] [PubMed] [Google Scholar]
- Milner TE. Adaptation to destabilizing dynamics by means of muscle cocontraction. Exp Brain Res. 2002;143:406–16. doi: 10.1007/s00221-002-1001-4. [DOI] [PubMed] [Google Scholar]
- Milner TE, Cloutier C. Compensation for mechanically unstable loading in voluntary wrist movement. Exp Brain Res. 1993;94:522–32. doi: 10.1007/BF00230210. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Nicholas MK, Hodges PW. Does anticipation of back pain predispose to back trouble? Brain. 2004;127:2339–47. doi: 10.1093/brain/awh248. [DOI] [PubMed] [Google Scholar]
- Newell KM, Carlton LG. Force variability in isometric responses. J Exp Psychol Hum Percept Perform. 1988;14:37–44. [PubMed] [Google Scholar]
- Nitz AJ, Peck D. Comparison of muscle spindle concentrations in large and small human epaxial muscles acting in parallel combinations. Am Surg. 1986;52:273–7. [PubMed] [Google Scholar]
- O'Brien PR, Potvin JR. Fatigue–related EMG responses of trunk muscles to a prolonged, isometric twist exertion. Clin Biomech. 1997;12:306–313. doi: 10.1016/s0268-0033(97)00013-2. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–6. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- Parnianpour M, Nordin M, Kahanovitz N, Frankel V. The triaxial coupling of torque generation of trunk muscles during isometric exertions and the effect of fatiguing isoinertial movements on the motor output and movement patterns. Spine. 1988;13:982–92. doi: 10.1097/00007632-198809000-00004. [DOI] [PubMed] [Google Scholar]
- Potvin JR, O'Brien PR. Trunk muscle co-contraction increases during fatiguing, isometric, lateral bend exertions. Possible implications for spine stability. Spine. 1998;23:774–80. doi: 10.1097/00007632-199804010-00006. [DOI] [PubMed] [Google Scholar]
- Preuss R, Fung J. Can acute low back pain result from segmental spinal buckling during sub-maximal activities? A review of the current literature. Man Ther. 2005;10:14–20. doi: 10.1016/j.math.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25:947–54. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26:724–30. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- Reeves NP, Cholewicki J. Modeling the human lumbar spine for assessing spinal loads, stability, and risk of injury. Crit Rev Biomed Eng. 2003;31:73–139. doi: 10.1615/critrevbiomedeng.v31.i12.30. [DOI] [PubMed] [Google Scholar]
- Reeves NP, Cholewicki J, Milner TE. Muscle reflex classification of low-back pain. J Electromyogr Kinesiol. 2005;15:53–60. doi: 10.1016/j.jelekin.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Reeves NP, Everding VQ, Cholewicki J, Morrisette DC. The effects of trunk stiffness on postural control during unstable seated balance. Exp Brain Res. 2006;174:694–700. doi: 10.1007/s00221-006-0516-5. [DOI] [PubMed] [Google Scholar]
- Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine. 1995;20:2645–51. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- Rogers EL, Granata KP. Disturbed paraspinal reflex following prolonged flexion-relaxation and recovery. Spine. 2006;31:839–45. doi: 10.1097/01.brs.0000206361.53451.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JP, Martin B, Gauthier GM, Mussa Ivaldi F. Effects of whole-body vibration on spinal reflexes in man. Aviat Space Environ Med. 1980;51:1227–33. [PubMed] [Google Scholar]
- Sherwood DE, Schmidt RA, Walter CB. The force/force-variability relationship under controlled temporal conditions. J Mot Behav. 1988;20:106–16. doi: 10.1080/00222895.1988.10735436. [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Newell KM. Variability and noise in continuous force production. J Mot Behav. 2000;32:141–50. doi: 10.1080/00222890009601366. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Baratta RV, Banks A, Freudenberger C, Zhou BH. Flexion-relaxation response to static lumbar flexion in males and females. Clin Biomech. 2003;18:273–9. doi: 10.1016/s0268-0033(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Sparto PJ, Parnianpour M, Marras WS, Granata KP, Reinsel TE, Simon S. Neuromuscular trunk performance and spinal loading during a fatiguing isometric trunk extension with varying torque requirements. J Spinal Disord. 1997;10:145–56. [PubMed] [Google Scholar]
- Stokes IA, Gardner-Morse M, Churchill D, Laible JP. Measurement of a spinal motion segment stiffness matrix. J Biomech. 2002;35:517–21. doi: 10.1016/s0021-9290(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Gardner-Morse M, Henry SM, Badger GJ. Decrease in trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine. 2000;25:1957–64. doi: 10.1097/00007632-200008010-00015. [DOI] [PubMed] [Google Scholar]
- Taimela S, Kankaanpaa M, Luoto S. The effect of lumbar fatigue on the ability to sense a change in lumbar position. A controlled study. Spine. 1999;24:1322–7. doi: 10.1097/00007632-199907010-00009. [DOI] [PubMed] [Google Scholar]
- Van Dieen JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine. 2003;28:834–41. [PubMed] [Google Scholar]


