Abstract
The original hepatitis B virus (HBV) large surface antigen gene was synthesized. In order to optimize the expression of this gene in tomato plants, the tobacco pathogenesis-related protein S signal peptide was fused to the 5′ end of the modified gene and the sequence encoding amino acids S, E, K, D, E, and L was placed at the 3′ end. The gene encoding the modified HBV large surface antigen under the control of a fruit-specific promoter was constructed and expressed in transgenic tomato plants. The expression of the antigen from transgenic plants was confirmed by PCR and reverse transcriptase PCR. Enzyme-linked immunoassays using a monoclonal antibody directed against human serum-derived HBsAg revealed that the maximal level of HBsAg was about 0.02% of the soluble protein in transgenic tomato fruit. The amount of HBsAg in mature fruits was found to be 65- to 171-fold larger than in small or medium fruits and leaf tissues. Examination of transgenic plant samples by transmission electron microscopy proved that HBsAg had been expressed and had accumulated. The HBsAg protein was capable of assembling into capsomers and virus-like particles. To our knowledge, this is the first time the HBV large surface antigen has been expressed in plants. This work suggests the possibility of producing a new alternative vaccine for human HBV.
Hepatitis B virus (HBV) is a major cause of acute and chronic hepatitis. HBV DNA was found in most of the newborns from hepatitis B surface antigen (HBsAg)-positive mothers (27). The current HBV vaccine is a biotechnology product that falls in the category of “subunit vaccines” and is made from yeast cells grown by fermentation. The vaccines have prevented numerous infections. However, administration of the vaccine intramuscularly causes some pain, and it is not widely accepted, especially for children. In addition, current vaccines have the safety limitations of syringe-and-needle vaccination programs. In recent years, a novel production system of vaccines—“edible vaccines” or “oral vaccines”—has been developed. Compared to traditional vaccines, oral vaccines can serve multiple immunization priorities and offer advantages, including simplicity of use, lesser expense, greater convenience for storage, an increase in compliance (as a result of increased comfort of delivery), enhanced immune responses at mucosal sites, and stimulation of humoral immunity.
So far, much has been achieved in production of oral vaccines and proteins by using transgenic plants (1, 4, 17). The results of tests involving humans who ingested transgenic potato and lettuce expressing recombinant HBsAG (rHBsAg) have been reported, and the special antibody was detected in the ser of volunteers who were given edible tissues of transgenic plants (14). As we know, potatoes and lettuce cannot be eaten raw in certain populations. However, the cooking process would destroy the target protein and affect its immunogenicity. Transgenic edible raw crops can be produced at low cost. The agricultural infrastructure of any given country can accommodate a program to grow the vaccine-producing plant, and food-processing technology would be used for product manufacture. In our study, we chose the tomato as the plant system for producing the recombinant antigen.
HBV is a mostly double-stranded DNA virus in the Hepadnaviridae family. The HBV genome includes four genes: pol, env, precore, and X, respectively, which encode the viral DNA polymerase, envelope protein, precore protein (which is processed to viral capsid), and protein X. The most recent vaccine is based on the cloned copies of the env gene in yeast. Studies of mRNA transcription suggest that this gene has the potential to code for three related proteins: (i) a protein of 226 amino acids, identified as a major protein constituent of the HBV envelope, termed the S protein; (ii) a protein with 55 additional amino acids at the N terminus, encoded by a portion of the env gene upstream of the S gene (pre-S1); (iii) a protein corresponding to the entire env gene (pre-S2). Three different proteins are produced by combining these proteins in different formations: large protein on the envelope (combination of the pre-S1, pre-S2, and S genes); middle-sized proteins on the envelope (pre-S2 and S genes); and the major component of HBsAg, the S protein. The extension polypeptides have been claimed to enhance the immunogenicity of the S antigen in eliciting anti-S antibody (9, 19, 20, 21, 22), suggesting that they may play an important role in the process of virus infection and the induction of a defensive host response.
Although many previous studies have shown that plant cells could express HBV vaccines, the studied oral vaccines for prevention of HBV produced in transgenic plants used mostly middle-sized proteins and the S protein (Table 1). Low achievement of the large protein in plant cells has prevented transgenic plants from becoming direct animal feed and the source of a commercially available HBV vaccine thus far. In this work, we attempted to improve HBV large surface antigen production in transgenic plants by many methods: for example, by using a plant bias codon gene, the plant Lys-Asp-Glu-Leu (KDEL) protein localization system, and a promoter that drives fruit-specific protein expression. The aim of our work was to investigate the feasibility of producing a new HBV oral vaccine with the HBV large surface antigen gene.
TABLE 1.
Proteins with application for human HBV vaccine and expressed by transgenic plants
| Yr of expression | Protein or peptide expressed | Plant expression system | Maximum recorded expression level in plantsa | Reference |
|---|---|---|---|---|
| 1992 | HBsAg | Tobacco | <0.01% TSP | 17 |
| 1997 | M and S protein | Potato | Not given | 7 |
| 1999 | HBsAg | Lupine (Lupinus spp.) | <0.15 μg/g FW | 14 |
| 2000 | HBsAg | Potato | <0.01 μg/g FW | 23 |
| HBsAg | Tomato | 8 μg/g FW fruit | 29 | |
| L protein | Tomato | Not detected | 29 | |
| 2002 | HBsAg | Kelp | 6.67 μg/g FW | 13 |
| HBsAg | Peanut | 0.032 TSP | 3 | |
| 2005 | HBsAg | Banana | 38 ng/g FW | 16 |
TSP, total soluble protein; FW, fresh weight.
MATERIALS AND METHODS
Chemical synthesis of HBV large surface antigen gene PRS-S1S2S.
The original HBV large surface antigen gene was synthesized based on the sequence of GenBank accession no. S20745. Oligonucleotides were synthesized using the oligo 1000 M DNA synthesizer (Beckman), and each oligonucleotide fragment was about 90 bp long. A total of 18 primers (Table 2) were designed according to the gene's synthetic strategy (Fig. 1), and about 20 nucleotide residues were overlapped between two neighboring primers (Table 2). Sequences encoding the tobacco pathogenesis-related protein S (PR-S) signal peptide (5) were fused to the 5′ end of the modified HBV large surface antigen gene. The sequence encoding amino acids S, E, K, D, E, and L was placed at the 3′ end of the HBV large surface antigen gene. The SEKDEL sequence was found to be sufficient for retention of secreted proteins in the endoplasmic reticulum (ER) (10). The synthesized products were deprotected with ammonium hydroxide and desalted on NAP 25 columns (Pharmacia). The method used to synthesize the PRS-S1S2S gene was the overlap extension PCR method (28). All primers were joined together in a single PCR (inner primer, 10 ng; outer primer, 100 to 200 ng). The PCR was performed at 94°C for 5 min for the first cycle, followed by 30 cycles of 94°C for 30 s, 62°C for 40 s, 72°C for 1 min, and 15 s. The accuracy of the synthetic PRS-S1S2S gene sequence was confirmed using the DNA sequencer ABI 377 (PE Applied Biosystems, Foster City, CA).
TABLE 2.
Oligonucleotides used to synthesize the PRS-S1S2S gene
| Oligonucleotide | Nucleotide sequence |
|---|---|
| HBSAG0 | 5′-TTG TTG GTC TTG CAG GCT GGT TTC TTC TTG TTG ACC AGA ATC TTG ACC ATC CCT CAG TCC TTG GAC TCC TGG TGG ACC TCC TTG AAC TTC-3′ |
| HBSAG1 | 5′-ATC TTC TCC AGA ATC GGT GAT CCT GCT TTG AAC ATG GAA AAC ATC ACC TCC GGT TTC TTG GGT CCT TTG TTG GTC TTG CAG GCT GGT TTC-3′ |
| HBSAG2 | 5′-GAG GTA GGA GAG TGG TTG GAG GTA GGG GAT TGG GAG TTT TGA CCC AAG CAG ACA GTA GTA CCT CCC AAG AAG TTC AAG GAG GTC CAC CAG-3′ |
| HBSAG3 | 5′-CCC TGC TGG TGG TTC CTC CTC CGG TGT CGT CAA CCC TGT CTT GAC CAC CGC TTC CCC TTT GTC CTC TAT CTT CTC CAG AAT CGG, TGA, TCC-3′ |
| HBSAG4 | 5′-AAG, ATG, AAC, AGG, AAA, ATG, ATG, AAT CTT CTC AAG CAC ATC CAT CTG TAA CCA GGG CAG GTA GGA GGG CAA GAG GTA GGA GAG TGG TTG GAG-3′ |
| HBSAG5 | 5′-AGC TAT GCA ATG GAA CTC CAC CAC CTT CCA CCA GAC CTT GCA AGA CCC TAG AGT CAG AGG TTT GTA CTT CCC TGC TGG TGG TTC CTC CTC-3′ |
| HBSAG6 | 5′-AAG GGC AGA CAG GCA ACA TAC CCT GGT AGT CCA ACA AGA CCA ACA AGA AGA TCA AGC ACA ACA GCA AGA TGA ACA GGA AAA TGA TGA ATC-3′ |
| HBSAG7 | 5′-TCC ACC AAC AGA CAG TCC GGT AGA CAA CCT ACC CCT TTG TCC CCT CCT TTG AGA AAC ACC CAC CCT CAA GCT ATG CAA TGG AAC TCC ACC-3′ |
| HBSAG8 | 5′-AGT ACC TTG AGC AGT GGT CAT GAC AGT TCT GCA AGG ACC GGT AGA GGT GGT AGA GGA ACC AGG GAT CAA AGG GCA GAC AGG CAA CAT ACC-3′ |
| HBSAG9 | 5′-TTT GCT GGG TTG GTC TCC TCA GGC TCA GGG TAT CTT GCA GAC CGT CCC TGC TAA CCC TCC TCC TGC TTC CAC CAA CAG ACA GTC CGG TAG-3′ |
| HBSAG10 | 5′-GAA GGG ATA GGG ATG CAG GTG CAG TTA CCG TCG GAA GGC TTG GTG CAA CAG CAG GAA GGG TAC ATA GAA GTA CCT TGA GCA GTG GTC ATG-3′ |
| HBSAG11 | 5′-ACC TGG CCT GAC GCT AAC AAG GTC GGT GCT GGT GCT TTC GGT TTG GGT TTC ACC CCT CCT CAC GGT GGT TTG CTG GGT TGG TCT CCT CAG-3′ |
| HBSAG12 | 5′-ACC AGC AAG GAC AAC CAG GAG AAT CTA GCG GAA GCC CAT TCC CAC AAG AAC TTA CCG AAA GCC CAG GAG GAA GGG ATA GGG ATG CAG GTG-3′ |
| HBSAG13 | 5′-CAC CAG TTG GAC CCT GCT TTC AGA GCT AAC ACC GCT AAC CCT GAC TGG GAC TTC AAC CCT AAC AAG GAC ACC TGG CCT GAC GCT AAC AAG-3′ |
| HBSAG14 | 5′-TAC CAC ATC ATC CAG ATG ACG GAC AAC CAG ACG GTA GGG GAC AAA CCG ACG AAC CAC TGG ACG AAA GGG ACC AGC AAG GAC AAC CAG GAG-3′ |
| HBSAG15 | 5′-TAC TTC GTC GCT GTG ACT CAC GCT GGT CAG AAC TTG TCC ACT TCT AAC CCT TTG GGT TTC TTC CCT GAC CAC CAG TTG GAC CCT GCT TTC-3′ |
| HBSAG16 | 5′-TGA GCT CTT ACA AAG GCA AGA AAG GGG ACA AGA TGG AGT ACA AGG AAG GAC CCC AGT ACC ACA TCA TCC AGA TGA CG-3′ |
| HBSAG17 | 5′-AAG GAT CCA ACA ATG GAC TTC TTG AAA TCT TTC CCA TTC TAC GCT TTC TTG TGC TTC GGT CAA TAC TTC GTC GCT GTG ACT CAC GCT-3′ |
FIG. 1.
Strategy used to synthesize the PRS-S1S2S gene by the overlap extension PCR method. Oligonucleotides of 60 bp were assembled by single-step PCR with 1.5 pmol of inner primers and 30 pmol of external primers.
Construction of plant expression vectors.
The fruit-specific promoter 2A11 (GenBank accession no. M87659) was amplified (forward, 5′-AAACTGCAGCCCTTTAAAAAGTATAGTCAATATTTAC-3′; reverse, 5′-AAAGGATCCGGTTTTGGATTAATTGCTAATTGATG-3′) from tomato genomic DNA and inserted into the PstI and BamHI site of a plant binary vector, pYPX143 (GenBank accession no. AY178047). The 1,245-bp DNA fragment containing the gene PRS-S1S2S was ligated into the vector pYPX143 between BamHI and SacI to form the HBV large surface antigen expression vector pYF9616 (Fig. 2).
FIG. 2.
Linear map of T-DNA region of pYF9616.
Plant transformation.
The pYF9616 plasmids were transformed into Agrobacterium strain LBA4404 cells by using the electroporation method (6). Tomato plants were transformed by a modified leaf disk cocultivation method using Agrobacterium tumefaciens harboring pYF9616 (11). Shoots were generated from transformed calls selected on medium containing 0.05 mg of kanamycin per ml and 0.25 mg of carbenicillin per ml. Shoots were rooted in medium containing 1 × 10−4 mg of 3-indole butyric acid per ml and 0.125 mg of carbenicillin per ml, transplanted to soil, and grown to maturity.
Screening of transgenic plants for PRS-S1S2S gene.
The transformed tomato plants were screened by PCR for the partial PRS-S1S2S fragment (300 bp) using the following primer pairs: forward, 5′-CCTGGTTACAGATGGATGTGCTTGAG-3′; reverse, 5′-AGCGGAAGCCCATTCCCACAAGAAC-3′. DNA extraction was carried out according to the method of Saunders (24).
Analysis of total RNA extracted from transgenic plants.
Total RNA from leaves of plants transformed with pYF9616 was isolated as described previously (18). The PRS-S1S2S mRNA was detected by reverse transcriptase PCR (RT-PCR) amplification using the Access RT-PCR system (Promega) (partial PRS-S1S2S amplification product, 300 bp; forward, 5′-CCTGGTTACAGATGGATGTGCTTGAG-3′; reverse, 5′-AGCGGAAGCCCATTCCCACAAGAAC-3′).
Analysis of protein from transformed tomato.
An HBsAg enzyme-linked immunosorbent assay (ELISA) kit was purchased from Huamei Biotechnology Co., Ltd. HBsAg standards were kindly provided by Y. Lu, Fudan-Yueda Biotechnology Company. Transgenic tomato plants were ground with liquid nitrogen and then incubated in phosphate-buffered saline (pH 7.0). Total soluble proteins containing expressed HBsAg were detected by ELISA and quantified with the standard curve establish by series dilution of HBsAg standards.
Transmission electron microscopy (TEM).
We viewed not only the mature tomato fruit extract but also the mature tomato fruit sections with immunogold labeling. On the one hand, the tomato fruit extract was bound with 1% bovine serum albumin onto copper grids. The trapped HBsAg particles were probed with monoclonal antibody to BALB/c mouse HBsAg (1:10) and secondary gold-labeled (5 nm) anti-mouse antibody (1:20). The grids were stained with phosphotungstic acid and viewed using a Philips Tecnai 12 transmission electron microscope. On the other hand, mature tomato fruit were excised, cut into 1-mm squares with a razor blade, fixed in 2.5% glutaraldehyde for 4 h at 4°C, washed, and dehydrated in a graduated ethanol series at 4°C, 100% acetone. The tissue was infiltrated with epon resin at 37°C and cured at 37°C for 12 h and then at 45°C for 48 h. Sections of about 60 nm in thickness were obtained by using a Reichert-Jung microtome. The sections were viewed by immunogold labeling, stained by uranyl acetate and lead lemon, and viewed on a Philips Tecnai12 electron microscope.
RESULTS
Chemical synthesis of HBV large surface antigen gene PRS-S1S2S.
The entire nucleotide sequence of the synthetic HBV large surface antigen gene PRS-S1S2S (GenBank accession no. S20745) is 1,245 bp in length. To improve protein secretion, a 72-bp sequence encoding the tobacco PR-S signal peptide was added to the 5′ coding regions of the modified HBsAg genes, and an 18-bp sequence encoding the ER retention signal SEKDEL was added to the C-terminal regions of modified HBsAg. For the modified gene, all codons were designed to be preferential to tomato plants. The G+C and A+T contents in the synthesized gene were balanced, and the predicted hairpin structures and motifs containing six consecutive A/Ts were eliminated (Fig. 3).
FIG. 3.
Nucleotide and deduced amino acid sequences of the HBV large surface antigen gene.
Transformation and regeneration of tomato.
The plasmid for expression of HBV large surface antigen in plants (pYF9616) (Fig. 2) was introduced into Agrobacterium tumefaciens and used in transformation experiments. Kanamycin incorporated into tissue culture medium allowed selection of transformed callus tissue, from which shoots and thereafter mature tomato plants were regenerated over a period of about 4 months. Thirteen independent kanamycin-resistant lines of transgenic plants were finally generated. All lines were transferred to a greenhouse for fruiting. Mature fruits of some lines bore few seeds. Such effects could result from insertion mutagenesis. F1 progenies of three lines were obtained.
RNA analysis.
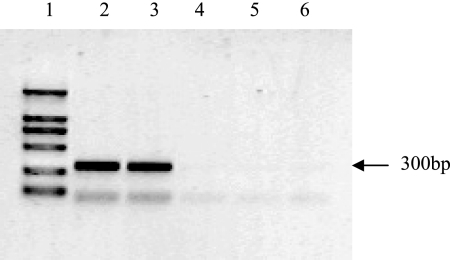
RT-PCR analysis was carried out to show the presence of an amplified product of the expected size (300 bp) in genomic DNA of tested PCR- and glucuronidase-positive plants (data not shown) which was absent in nontransformed plants. According to the protocol of a reverse transcription system kit (Promega), the s1s2s gene was proved to be transcribed in all transgenic plants but not in nontransgenic plants (Fig. 4).
FIG. 4.
Detection (using RT-PCR) of s1s2s gene transcription in transgenic tomatoes. Lane 1, DL2000 molecular weight marker; lanes 2 and 3, RNA from transgenic tomato; lanes 4 and 5, PCR amplification without reverse transcriptase reaction as control for DNA contamination; lane 6, RNA from nontransgenic tomato.
Immunodetection of HBV large surface antigen.
The ELISA method was used to detect and quantitatively analyze the target protein. rHBsAg levels in leaves and fruits from a representative tomato plant line of several tactic lines are presented in Table 3. The results indicated that the expression levels of rHBsAg varied in leaves and fruits in the same plant. Apparently, rHBsAg showed high levels in mature fruit, while leaves and immature fruit had low levels. The amount of HBsAg in mature fruit was found to be 65- to 171-fold higher than in small or medium fruits and leaf tissues. rHBsAg protein levels in immature fruits and leaves were in a similar range, and variation was observed among the mature fruits in different plants. The expression level of rHBsAg also appeared much lower in other organs, including roots, flowers, and stems, than in mature fruit (Table 4).
TABLE 3.
Expression of HBsAg in different transgenic tomato lines
| Plant no.a | Organ | Amt of HBsAg (ng/ml) | TSPb (mg/ml) | HBsAg/TSP ratio (10−6) | Amt of HBsAg (fresh wt) (ng/g) |
|---|---|---|---|---|---|
| 1# | Small fruit | 2.12 | 1.49 | 1.42 | 2.25 |
| Medium fruit | 2.35 | 0.30 | 7.81 | 2.23 | |
| Mature fruit | 156.84 | 2.12 | 73.90 | 266.63 | |
| Leaf | 1.76 | 4.29 | 0.41 | 2.78 | |
| 2# | Small fruit | 1.43 | 1.40 | 1.02 | 1.75 |
| Medium fruit | 1.55 | 0.28 | 5.52 | 1.80 | |
| Mature fruit | 101.29 | 0.51 | 200.00 | 168.82 | |
| Leaf | 1.38 | 4.81 | 0.29 | 2.03 | |
| 3# | Small fruit | 2.23 | 1.50 | 1.48 | 3.04 |
| Medium fruit | 2.47 | 0.29 | 8.46 | 2.96 | |
| Mature fruit | 381.43 | 2.66 | 143.00 | 523.53 | |
| Leaf | 3.18 | 5.70 | 0.56 | 3.82 | |
| CK | Small fruit | 1.42 | |||
| Medium fruit | 0.47 | ||||
| Mature fruit | 1.71 | ||||
| Leaf | 5.10 |
Note that 1#, 2#, and 3# were different plants of the same transgenic tomato line; CK was a nontransformed plant grown in the greenhouse.
TSP, total soluble protein.
TABLE 4.
Expressed target protein
| Organ | Amt of HBsAg (ng/ml) | TSPa (mg/ml) | HBsAg/TSP ratio (10−6) | Amt of HBsAg (fresh wt) (ng/g) |
|---|---|---|---|---|
| Leaf | 3.02 | 5.10 | 0.59 | 3.90 |
| Root | 3.18 | 0.56 | 5.68 | 2.65 |
| Stem | 2.12 | 0.62 | 3.45 | 2.47 |
| Flower | 2.00 | 1.75 | 1.14 | 2.85 |
| Mature fruit | 300.02 | 1.98 | 151.53 | 400.01 |
TSP, total soluble protein.
TEM.
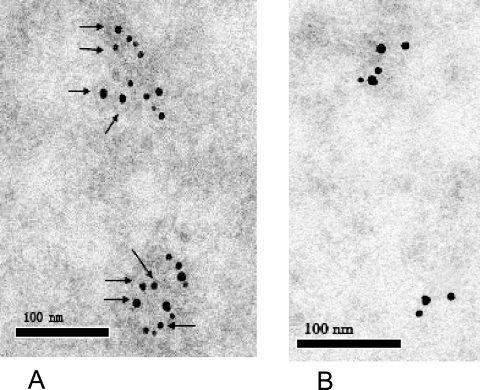
The foregoing results suggested that rHBsAg can assemble into particles which are about 17 nm in diameter. To determine whether the human HBV surface large antigen in tomato plants also assembled into similar particles, the protein was immunotrapped in the plant extracts using the immunogold labeling method. Gold particles can be observed attached to plant-produced particles, binding to the putative capsomers, which are about 17 nm in diameter (Fig. 5A). None of these kinds of particles was observed for nontransgenic control extracts (Fig. 5B). It was proved that the recombined HBV large surface antigen could retain the capacity for self-association and has the physical properties of human serum-derived HBsAg, which is highly immunogenic in the particle form.
FIG. 5.
Electron micrographs of immunogold-labeled capsomers of human HBV large surface antigen in s1s2s transgenic mature tomato fruit (A) and nontransformed mature tomato fruit (B). Arrows indicate full-size particles of human HBV large surface protein. Bars, 100 nm.
In order to determine where the recombined particles assemble in the plant cell, we observed the immunogold labeled tomato fruit section by TEM. Gold particles were observed near the ER. This is similar to the production of rHBsAg in the leaves of transgenic potato (15). Microscopic examination of cells of nontransgenic tomato mature fruit did not reveal any gold particles near the ER. We therefore conclude that transgenic plant cells may accumulate recombinant HBV surface large antigen near the ER.
DISCUSSION
The data presented in this paper prove that a human HBV surface large antigen fusion gene could be expressed in tomato efficiently. To our knowledge, this is the first report to demonstrate that the human HBV surface large antigen can be synthesized in plants.
For transgenic plants, a large proportion of capsomers can be observed by electron microscopy in extracts and fruit sections. This result is different from that reported by Imamura et al., who thought that the large S gene could not be assembled into particles in Saccharomyces cerevisiae (12). Zhao et al. also attempted to express the human HBV surface large antigen in tomato (29). No spherical particles 22 nm in diameter were observed by electron microscopy, nor were the specific proteins examined by the ELISA method in the extracts and sections of transgenic tomato fruit. This may have been caused by an inefficient system of expression of the human HBV surface large antigen gene.
Transgenic plants expressing recombinant antigens have been developed successfully since the method was first described by Mason et al. (17). A corollary of this research is to develop subunit vaccines which are produced in edible plant products, such that the plant-derived vaccine can be ingested directly without prior purification or processing. This appears to be a very promising alternative to other methods for expressing recombinant protein. Nevertheless, a number of questions and challenges still remain to be solved. Also, the method's main disadvantage is low yields of expressed antigen. Next are several risks during the production and delivery stages of this technology, with potential impacts on the environment and on human health. Risks to the environment include gene transfer and exposure to antigens or selectable marker proteins. Risks to human health include oral tolerance, allergy, inconsistent dosage, worker exposure, and unintended exposure to antigens or selectable marker proteins in the food chain. These risks are controllable through appropriate regulatory measures at all stages of production and distribution of a potential plant-made vaccine. Successful use of this technology is highly dependent on stewardship and active risk management by the developers of this technology and through quality standards for production, which will be set by regulatory agencies.
Various approaches other than use of a fruit promoter have been suggested to increase the expression levels in transgenic plants. These include codon optimization and use of a specific promoter. Mason et al. reported that the HBsAg concentration in engineered tobacco was 0.01% of the total soluble protein (17). In this report, we describe the use of the fruit-specific promoter 2A11 to increase the level of foreign antigen protein and to show greater expression efficiency (26). The highest value was 0.02% of total soluble protein (Table 3). It was also very efficient for the expression of heterogeneous protein in transgenic plants to target the protein to different subcellular compartments, such as the chloroplasts of the ER, or potentially membrane-anchoring regions, where there is better accumulation and usually less proteolytic activity (2). The KDEL is commonly found at the C terminus of soluble proteins in the ER, and it contributes to protein localization by interaction with a receptor that recycles between the Golgi complex and the ER (8). The KDEL signal is sufficient for stable protein accumulation in the plant ER (23). Stoger et al. (25) found that single-chain antibody fragment levels were 6 to 14 times higher in cells transformed with the single-chain antibody fragment gene containing the KDEL than those transformed with the same gene without the KDEL. In our study, the tobacco PR-S signal (7) was added in the N terminus and the KDEL ER retention signal at the C terminus encoded by the HBV large surface antigen gene.
The results of this work suggest the possibility of producing a new, alternative vaccine for human HBV. This alternative vaccine will be less expensive and more convenient to store than traditional vaccines. This work also gave us properties dedicated to developing technologies for a high-immunogenicity and low-cost oral vaccine for the developing world, though this is only a successful first step in a long-term project. Much more work needs to be done: for example, studying the immunogenicity of the recombinant large surface antigen, the stability of foreign genes in further generations, the security of transgenic plants, and so on. In future work, we will start orally immunizing mice with transgenic tissues to test the immunogenicity of the tomato-derived recombined human HBV large surface antigen and study the stability of foreign genes in further generations.
Acknowledgments
We are grateful to Shanghai YongYe Bio-engineering Co., Ltd., China, for providing instruments and technical help and to the Testing Center of Yangzhou University for electron microscopy.
This research was supported by the Shanghai Key Laboratory of Agricultural Genetics and Breeding, People's Republic of China (shagb2005-01).
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Arakawa, T., D. K. Chong, and W. H. Langridge. 1998. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 16:292-297. [DOI] [PubMed] [Google Scholar]
- 2.Awram, P., R. C. Gardner, R. L. Forster, and A. R. Bellamy. 2002. The potential of plant viral vectors and transgenic plants for subunit vaccine production. Adv. Virus Res. 58:81-124. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H. Y., J. Zhang, Y. Gao, H. L. Du, Y. Ma, W. Z. Zheng, and N. S. Xia. 2002. Transforming HBsAg into peanut and detection of its immunogenicity. Lett. Biotechnol. 4:245-250. [Google Scholar]
- 4.Chikwamba, R., J. Cunnick, D. Hathaway, J. McMurry, H. Mason, and K. Wang. 2002. A functional antigen in a practical crop: LT-B producing maize protects mice against Escherichia coli heat labile enterotoxin (LT) and cholera toxin (CT). Transgenic Res. 11:479-493. [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen, B. J. C., R. A. M. Hooft Van Huijduijnen, and J. F. Bol. 1986. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. Nature 321:531-532. [DOI] [PubMed] [Google Scholar]
- 6.Dower, S. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehsani, P., A. Khabiri, and N. N. Domansky. 1997. Polypeptides of hepatitis B surface antigen produced in transgenic potato. Gene 190:107-111. [DOI] [PubMed] [Google Scholar]
- 8.Frigerio, L., A. Pastres, A. Prada, and A. Vitale. 2001. Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13:1109-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heermann, K. H., U. Goldmann, W. Schwartz, T. Seyffarth, H. Baumgarten, and W. H. Gerlich. 1984. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 52:396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman, E. M., B. W. Tague, L. M. Hoffman, S. E. Kjemtrupp, and M. J. Chrispeels. 1990. Retention of phytohemagglutinin with carboxy terminal tetrapeptide KDEL in the nuclear envelope and the endoplasmic reticulum. Planta 182:305-312. [DOI] [PubMed] [Google Scholar]
- 11.Horsch, R. B., J. E. Fry, N. L. Hoffman, D. Eichholtz, S. G. Rogers, and R. T. Fraley. 1985. A simple and general method for transferring genes into plants. Science 227:1229-1231. [DOI] [PubMed] [Google Scholar]
- 12.Imamura, T., M. Araki, A. Miyanohara, J. Nakao, H. Yonemura, N. Ohtomo, and K. Matsubara. 1987. Expression of hepatitis B virus middle and large surface antigen genes in Saccharomyces cerevisiae. J. Virol. 11:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, P., S. Qin, and T. Chengkui. 2002. Expression of hepatitis B surface antigen gene (HBsAg) in Laminaria japonica (Laminariales, Phaeophyta). Chin. Sci. Bull. 17:1438-1440. [Google Scholar]
- 14.Kapusta, J., A. Modelska, M. Figlerowicz, T. Pniewski, M. Letellier, O. Lisowa, V. Yusibov, H. Koprowski, A. Plucienniczak, and A. B. Legocki. 1999. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 13:1796-1799. [DOI] [PubMed] [Google Scholar]
- 15.Kong, Q. X., L. Richer, Y. F. Yang, C. J. Arntzen, H. S. Mason, and Y. Thanavala. 2001. Oral immunization with hepatitis B suface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 981:11539-11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar, G. B. S., T. R. Ganapathi, C. J. Revathi, L. Srinivas, and V. A. Bapat. 2005. Expression of hepatitis B surface antigen in transgenic banana plants. Planta 3:484-493. [DOI] [PubMed] [Google Scholar]
- 17.Mason, H. S., D. M. Lam, and C. J. Amtxen. 1992. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 89:11745-11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason, H. S., and J. E. Mullet. 1990. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell 2:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel, M. L., P. Pontisso, E. Sobczak, Y. Malpiece, R. E. Streeck, and P. Tiollais. 1984. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerized human serum albumin. Proc. Natl. Acad. Sci. USA 81:7708-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milich, D. R., G. B. Thornton, A. R. Neurath, S. B. Kent, M. L. Michel, P. Tiollais, and F. V. Chisari. 1985. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science 228:1195-1199. [DOI] [PubMed] [Google Scholar]
- 21.Milich, D. R., M. K. McNamara, A. McLachlan, G. B. Thornton, and F. V. Chisari. 1985. Distant H-2-linked regulation of T-cell response to the pre-S and S region of the same hepatitis B surface antigen polypeptide allows circumvention of nonresponsiveness to the S region. Proc. Natl. Acad. Sci. USA 82:8168-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath, A. R., S. B. H. Kent, and N. Strick. 1984. Location and chemical synthesis of a pre-S gene coded immunodominant epitope of hepatitis B virus. Science 224:392-395. [DOI] [PubMed] [Google Scholar]
- 23.Richter, L. J., Y. Thanavala, C. J. Arntzen, and H. S. Mason. 2000. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 18:1167-1171. [DOI] [PubMed] [Google Scholar]
- 24.Saunders, G. W. 1993. Gel purification of red algal genomic DNA: an inexpensive and rapid method for the isolation of polymerase chain reaction-friendly DNA. J. Phycol. 29:251. [Google Scholar]
- 25.Stoger, E., S. Williams, P. Christou, R. E. Down, and J. A. Gatehouse. 1999. Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin; GNA) in transgenic wheat plants: effects on predation by the grain aphid Sitobion avenae. Mol. Breed. 5:65-73. [Google Scholar]
- 26.Van Haaren, M. J. J., and C. M. Houck. 1991. Strong negative and positive regulatory elements contribute to the high-level fruit-specific expression of the tomato 2A11 gene. Plant Mol. Biol. 17:615-630. [DOI] [PubMed] [Google Scholar]
- 27.Wang, S. P., D. Z. Xu, Y. P. Yan, M. Y. Shi, R. L. Li, J. X. Zhang, G. Z. Bai, and J. X. Ma. 2000. Hepatitis B virus infection status in the PBMC of newborns of HBsAg positive mothers. World J. Gastroenterol. 6(Suppl. 3):58. [Google Scholar]
- 28.Xiong, A. S., Q. H. Yao, R. H. Peng, X. Li, H. Q. Fan, Z. M. Cheng, and Y. Li. 2004. A simple, rapid, high-fidelity and cost-effective PCR-based two-step DNA synthesis method for long gene sequences. Nucleic Acids Res. 32:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, C. H., R. Wang, C. S. Zhao, G. L. Wang, and P. Tian. 2000. Expression of human HBV surface antigen gene with and without preS in transgenic tomato. Nongye Shengwu Jishu Xubao 8:85-88. [Google Scholar]