Abstract
The Bradyrhizobium japonicum fixRnifA operon is transcribed from two promoters: fixRp1, a −24/−12 promoter recognized by the σ54-holoenzyme form of the RNA polymerase, and fixRp2, a −35/−10 promoter that is transcribed by a second, unidentified, form of RNA polymerase holoenzyme. The fixRp1 promoter is autoregulated during microaerobiosis by NifA, whereas fixRp2 is also activated, but by a different regulatory protein. The main transcription start sites for these promoters are just two nucleotides apart, such that the conserved −12 and −10 regions of fixRp1 and fixRp2, respectively, must overlap each other, whereas the −24 and −35 regions lie one DNA helical turn apart. Using in vivo genomic dimethyl sulfate and KMnO4 footprinting, we showed that the promoter region is differentially protected, depending upon which holoenzyme is bound. Mutagenesis analyses indicated that positions from −12 to −14 are critical for the activity of both promoters, whereas mutations at −10 and −11 affected mainly fixRp2 expression. When the sequence of the putative −35 region of fixRp2 was modified to match the putative consensus, expression from this promoter was increased 3-fold and the reactivity toward KMnO4, but not the transcriptional start site, moved two nucleotides further upstream, indicating that the altered promoter forms a different open complex. Additionally, we detected NifA-dependent methylation protection of two atypical NifA binding sites and protection of guanine −75. The latter residue is located in a region critical for fixRp2 promoter activation. The results present direct physical evidence of the complexity of the organization, regulation, and function of the fixRnifA promoter region.
Keywords: σ54, open complex, transcription, protein–DNA interactions
In Bradyrhizobium japonicum, the root nodule endosymbiont of soybean plants, as in most of the nitrogen fixing proteobacterial species, genetic control of free-living (nif) and symbiotic (fix) nitrogen fixation gene expression is exerted by NifA. This protein belongs to the enhancer-binding protein (EBP) family of regulators that activate transcription from promoters recognized by RNA polymerase holoenzyme with the alternative sigma factor σ54 (Eσ54) (reviewed in refs. 1–3). Regulation of nif and fix gene expression is finely controlled by oxygen, which regulates both the expression and the activity of NifA (4). Promoters recognized by Eσ54 are unique in having two DNA boxes unusually close together, centered at −12 and −24 nucleotides upstream from the transcription start site, instead of the more common −10 and −35 boxes found in most of the bacterial promoters (5). Eσ54 binds to these sequences and forms a stable closed complex that is isomerized to the transcriptionally active open promoter complex only by the appropriate EBP, in a process that requires ATP hydrolysis (6–9). To activate transcription the EBP binds to enhancer-like elements located far from the promoter, typically more than 100 bp upstream (10–12). Genetic and in vivo footprinting analyses have shown that NifA binds to DNA sequences of the type TGT-N10-ACA, called upstream activator sequences (UASs) (11, 13). The nif and fix genes have up to three UASs, and the number of these elements in a given promoter contributes to the level of NifA-mediated activation (14, 15).
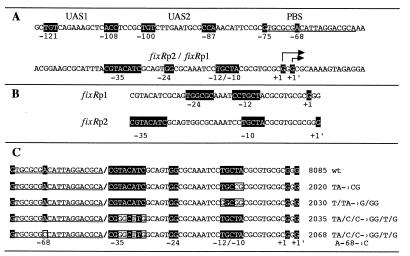
The B. japonicum NifA protein is encoded in the fixRnifA operon. This operon is positively autoregulated under anaerobiosis and, unexpectedly, it is also expressed under aerobic conditions, albeit at a lower level (16, 17). Deletion, primer extension, and mutagenesis analyses have shown that a region around position −68 is required for the aerobic expression (16, 17). Gel retardation experiments, using cell extracts, showed that this region is the binding site for a protein (17). We have previously shown that the pattern of expression of fixRnifA is due to the presence of two overlapping promoters: fixRp1, which belongs to the −24/−12 class, and fixRp2, which shares sequence identity with the −35 and −10 regions found in a set of other B. japonicum promoters (16). The nucleotide sequence of the latter promoters does not show similarity to any other known bacterial promoter and probably represents a new type of promoter.
Primer extension analyses showed that fixRp1 directs the synthesis of a transcript, called p1, that starts 12 nucleotides downstream of the −12 region. In addition to σ54, p1 is dependent on NifA, and it is observed only at low oxygen tensions. Transcripts originating from fixRp2 start at two sites: one coincides with the start site for p1, whereas the more abundant transcript, designated p2, initiates just 2 nucleotides further downstream (16). Expression from fixRp2 requires the integrity of the upstream −68 promoter region but it is independent of σ54 and NifA. This promoter is expressed under aerobic and anaerobic conditions but it is not expressed in root nodule bacteroids (16). Mutations in the −24 region of the σ54-dependent fixRp1 promoter impair only the p1 transcript, showing that this region is not required for the expression of fixRp2. Conversely, mutants in the conserved −12 region of the fixRp1 promoter do not show any transcript, suggesting that these changes simultaneously disrupt the overlapping −10 region of the fixRp2 promoter. In agreement with this observation, in a strain lacking σ54 the expression from the fixRp2 promoter is enhanced severalfold, suggesting that, in the wild-type strain, the two RNA polymerase holoenzymes compete for binding to the same promoter region (16).
In this report we analyze the in vivo chromosomal occupancy and isomerization of fixRnifA wild-type and mutant promoters, by using dimethyl sulfate (DMS) and KMnO4, respectively. Results establish that the two RNA polymerase holoenzymes bind differently to a common promoter region and that fixRp1 is completely within the fixRp2 promoter. We also show NifA-dependent protection of two imperfect UASs at −100 and −121, and protection by a second regulatory protein of a guanine residue in the −68 region.
MATERIALS AND METHODS
Bacterial Strains and Plasmids.
All the strains used in this work were constructed by chromosomal integration by means of homologous recombination of the appropriate fixR-lacZ fusion (16). Chromosomal integration results in the duplication of the fixRnifA promoter region up to the site of the lacZ gene fusion (codon 8 of fixR). Strain 8085 contains the wild-type fixR-lacZ fusion in the wild-type B. japonicum 110spc4 strain, whereas strain 8091 has an A-to-C transversion at position −68 (18). In strains A8085 and N8085 the fixR-lacZ fusion was integrated into the nifA mutant strain A9 (19), or into the rpoN1/rpoN2 double mutant strain N50–97 (20), respectively.
Growth Conditions.
B. japonicum cultures were normally grown aerobically in PSY medium (18) at 30°C with the following antibiotics: kanamycin at 100 μg/ml, tetracycline at 50 μg/ml, streptomycin at 100 μg/ml, and spectinomycin at 100 μg/ml. Anaerobic cultures were grown in yeast extract/mannitol medium with 10 mM KNO3 as the electron acceptor.
DMS Genomic Footprinting.
Anaerobic cultures (160 ml) were grown until an OD540 of 0.5–0.6, and DMS was added to a final concentration of 0.1%. After 1 min the cells were collected by centrifugation and washed twice with saline phosphate solution (11). When required, the cultures were preincubated 10 min with rifampicin (200 μg/ml) as previously reported (5). The methylated DNA was purified according to standard procedures and was cleaved by incubation with piperidine as described (21). The methylation pattern was obtained by primer extension using 9 μg of DNA, 0.5 pmol of the appropriate 32P 5′-end-labeled oligonucleotide [lac4 (16) or fixR upstream (5′-GTGAAAGCGAACGCGGGC-3′)]. These oligonucleotides prime 119 nucleotides downstream or 204 nucleotides upstream from the fixR-lacZ +1, respectively, and were extended by linear amplification using Taq DNA polymerase (GIBCO/BRL) by 20 cycles (1 min at 92°C, 1 min at 55°C, and 90 sec at 72°C).
Potassium Permanganate Reactivity Analysis.
Anaerobically grown 160-ml cultures were incubated with rifampicin for 10 min before exposure to KMnO4 (10 mM), as previously reported (5). The DNA was isolated as described above and the oxidized pyrimidine residues were detected by using the same conditions as for the methylated DNA. Primer extension products derived from the DMS or KMnO4 modifications were separated by electrophoresis on 6% polyacrylamide gels and exposed on hyperfilm β-Max films (Amersham) or scanned in a Molecular Dynamics PhosphorImager.
Site-Directed Mutagenesis.
The −10 and −35 promoter regions were mutagenized by a direct oligonucleotide method based on PCR (22). A degenerate oligonucleotide with a mixture of the four nucleotides at positions −10, −11, and −14 (CGCAAATCCNGCNNCGCGTGCG) was used to generate the double and triple mutants described here. For the construction of the −35 mutant promoter an oligonucleotide with four substitutions was synthesized (GCATTTACGGGCTTGGCAGTGGC). The oligonucleotides were extended by using as template plasmid pRJ7211, which contains a fixR-lacZ fusion, or total DNA of strain 8091, which carries a fixR A−68-to-C promoter mutation also fused to lacZ, and an oligonucleotide that primes in the coding region of the lacZ gene. These PCR products were used as megaprimers and extended further downstream, as reported (22). The digested products were used to replace the wild-type 331-bp SmaI–BamHI promoter fragment, and the mutations were confirmed by sequencing the entire cloned fragment.
Chromosomal Integration of the Mutant fixR-lacZ Fusions.
To determine the effect of the different fixR mutations on promoter performance, the various promoter mutations were cloned into the suicide plasmid pSUP202. This plasmid carries a tetracycline-resistance gene and cannot replicate in B. japonicum. Therefore when it is introduced by conjugation into B. japonicum and selection for tetracycline resistance is applied, integration by homologous recombination is achieved. The four mutant fixR-lacZ fusion derivatives were cloned into EcoRI/NcoI-digested pSUP202, as EcoRI–DraI fragments, and conjugated into the B. japonicum wild-type strain, and tetracycline-resistant derivatives were isolated. To confirm the site of recombination (the plasmids could recombine at low frequencies between the promoter and the lacZ gene, yielding a wild-type fixR-lacZ fusion and a mutant promoter in front of the fixRnifA operon) total DNA from all the transconjugant strains was purified, the fixR-lacZ promoter region was selectively amplified, and the resulting PCR products were sequenced. In all the strains probed the recombination event took place at the desired location, rendering the mutant promoters in front of the transcriptional fixR-lacZ fusions. The β-galactosidase activity of each fusion was determined as described (16).
Primer Extension Analysis.
To determine the transcription start sites for the mutant fixR-lacZ promoters, total RNA from the B. japonicum transconjugant strains was purified and analyzed by primer extension, as previously described (16).
RESULTS AND DISCUSSION
NifA-Dependent DMS Protection of Two Imperfect UASs.
In vivo fixR-lacZ expression analyses have shown that NifA autoregulates its expression during microaerobic growth (16, 17). This activation is partially dependent on sequences upstream from the promoter (17), suggesting that NifA must interact with the DNA. Fig. 1A shows the nucleotide sequence of the fixRnifA promoter region. Two elements resembling NifA binding sites (UAS), located at −100 (TGT-N10-CCA) and −121 (TGT-N10-ACC) nucleotides upstream from the σ54-dependent transcription start site, are indicated. These sequences have five of the six positions that conform to the canonical UAS elements (10, 11). To find out whether these putative UAS elements are contacted by NifA, we carried out in vivo genomic DMS protection experiments in wild-type and nifA− backgrounds carrying chromosomally integrated fixR-lacZ fusions.
Figure 1.
Structure of the B. japonicum fixRnifA promoter region. (A) Nucleotide sequence of the fixRnifA upstream promoter region, showing the putative NifA binding sites (UAS1, centered at −121, and UAS2, centered at −100), and the protein binding site (PBS) in the −68 region. The overlapping promoter region is also shown (fixRp2/fixRp1). +1 indicates the start of the σ54-dependent p1 transcript; +1′ indicates the major transcription start site, p2, for the fixRp2 transcript. (B) Positions presumed to serve as conserved sequences for each promoter. (C) Mutations constructed at the fixRnifA promoter region. Relevant positions for each element are shaded, whereas positions in boxes indicate nucleotide substitutions in the mutant promoters.
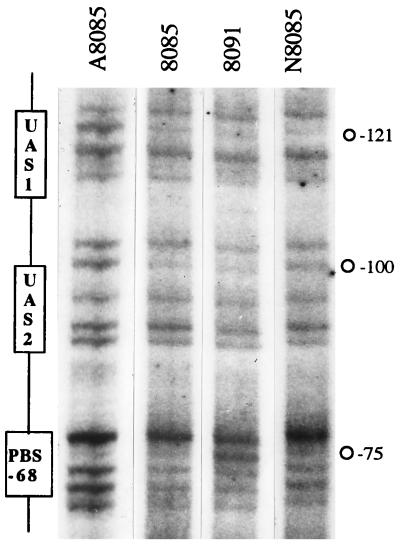
The rationale for doing the footprinting experiments directly over genomic DNA is derived from previous observations which established that the complex pattern of fixRnifA gene expression is detected only in chromosomally integrated fixR-lacZ fusions (17). If the fusions are plasmid-born, autoactivation by NifA is obscured (23), probably by a mechanism resembling the multicopy inhibition of nitrogen fixation by plasmid-born nif promoter sequences observed in Klebsiella pneumoniae (24). Genomic in vivo modified DNA was isolated and used as template for cycling primer extension with Taq DNA polymerase using a 5′- 32P-end-labeled synthetic oligonucleotide. With this method we were able to directly analyze the in vivo reactivity toward DMS of a discrete zone of the chromosomal DNA, and the influence that bound proteins have in such reactivity. Fig. 2 shows the pattern of cleaved products of the fixRnifA upstream regulatory region after exposure of anaerobically grown 8085 (wild type, lane 2), A8085 (nifA−, lane 1), 8091 (A−68 to C in wild-type background, lane 3), and N8085 (rpoN1−/rpoN2−, lane 4) strains to DMS. In the wild-type strain diminished products corresponding to positions −100 and −121 were observed compared with the nifA− strain, indicating a NifA-dependent protection from methylation of guanine residues of the TGT motifs of both putative UASs. Strain 8091, which does not express the fixRp2 promoter, showed a protection pattern similar to that of the wild-type strain, whereas strain N8085 showed a clear protection of UAS1 and a partial protection of UAS2. Thus, the protection of the UASs by NifA is independent of the activity of the promoters.
Figure 2.
Genomic DMS footprinting analysis of the B. japonicum fixRnifA upstream promoter region. The B. japonicum fixRnifA upstream promoter DNA region, integrated on the chromosome as a fixR-lacZ fusion, was analyzed by DMS footprinting in anaerobic cultures of strains A8085 (nifA− background), 8085 (wild-type background), 8091 (mutant A−68 to C promoter in wild-type background), and N8085 (rpoN1−/rpoN2− background). Primer extension products, obtained by linear amplification of the genomic DNA with Taq DNA polymerase, were separated by gel electrophoresis and detected by autoradiography. Guanine residue −121 of the top DNA strand was protected from methylation in all the strains except in the nifA− background, whereas guanine −100 was clearly protected in the 8085 and 8091 strains, and less protected in strain N8085. Guanine residue −75 of the protein binding site (PBS) is also shown (see Fig. 3).
The above results show that the requirement for upstream regions for activation by NifA is due to the presence of bona fide UASs, and that the binding of this protein is independent of the expression of the promoters. Protection of a UAS that does not fully match the consensus has previously been reported for the nifHc gene of Rhizobium etli (25) and the nifJ gene of K. pneumoniae (26). However, these observations have been made in heterologous systems having nifA expressed from a strong promoter on a multicopy plasmid, whereas in our experiments the only source of NifA was the chromosomal nifA gene. To our knowledge this is the first report in which the chromosomal occupancy of a bacterial enhancer has been demonstrated.
Methylation Protection of the −68 Region.
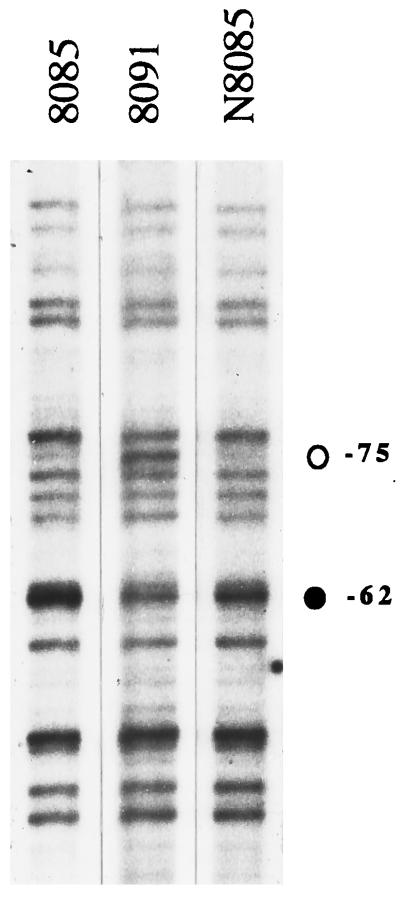
The fixRp2 promoter is expressed regardless of the nitrogen and carbon sources and it is only partially regulated by oxygen; however it is not expressed in 30-day soybean root nodule bacteroids (16). The regulatory mechanism controlling this expression pattern has not been fully determined. However, genetic analysis showed that the integrity of the upstream −68 region is essential for expression from the fixRp2 promoter. A single A-to-C transversion at position −68 abolishes fixRp2 expression (16, 17). These observations suggest that the fixRp2 promoter is positively regulated by a protein that binds to the −68 region. To find out whether this region is protected from methylation in vivo we performed genomic DMS footprinting experiments over the wild-type promoter in different genetic backgrounds and compared them with the promoter bearing the A−68-to-C transversion. Fig. 3 shows the methylation pattern of this region obtained from anaerobically grown 8085, 8091, and N8085 strains (see also strain A8085 in Fig. 2, lane 1). Compared with the strain having the A−68-to-C mutation (8091), a remarkable protection of G −75 and also hypermethylation of G −62 were observed in the strains with the wild-type promoter region, in all the genetic backgrounds tested. This result shows that the −68 region is a strong protein-binding site that extends at least from −68 to −75, and that the A−68-to-C mutation drastically impairs the in vivo binding of the activator protein, as suggested by the gel retardation experiments.
Figure 3.
Genomic DMS footprinting analysis of the B. japonicum fixRnifA −68 promoter region. The in vivo methylation pattern of the −68 region obtained from anaerobically grown 8085, 8091, and N8085 strains is shown. Protection of G −75 (○) and hypermethylation of G −62 (•) are indicated.
Interestingly, the nucleotide sequence of the −68 region showed a palindromic motif (TGCG-N11-CGCA), from −74 to −56, starting just beside the protected −75 guanine and encompassing the −68 position. Additional mutations in this region are necessary to define unambiguously the extent of the protein-binding site.
Mutagenesis Analysis of the fixRnifA Promoter Region.
On the basis of the comparison of the nucleotide sequence upstream of the transcription start site of the glnA, fbcFH, and fixRp2 genes of B. japonicum, we proposed a new type of promoter with no sequence similarity to any other known bacterial promoter (Fig. 1B) (16). Because of the diverse functions accomplished by these gene products we proposed that this could be the “housekeeping” B. japonicum promoter, recognized by a σ70 homologue. Hennecke and collaborators (27, 28) have recently determined the transcription start for the 16S ribosomal RNA. They found a motif highly resembling the consensus −35/−10 boxes for σ70 holoenzymes at the right distance from the transcription start site (TTGACA-N17-TATAAc-N7-+1), suggesting that the typical housekeeping promoter does exist in this organism. Moreover, they proposed that the glnA and fbcFH promoters have weak sequence similarity to the canonical −35/−10 promoters (27). Thus, although the fixRp2 promoter does not show sequence similarity to the canonical −35/−10 promoters for σ70 holoenzymes it could be transcribed by the most abundant (housekeeping) RNA polymerase.
To determine which nucleotides are critical for fixRp2 we constructed a set of mutations around the conserved GC dinucleotide of the fixRp1 −12 region (Fig. 1C), and integrated them by homologous recombination into the chromosome of the wild-type B. japonicum strain (see Materials and Methods). Table 1 shows the expression of each of the fixR-lacZ fusions in aerobic and anaerobic cultures. A triple mutant in which the −14, −11, and −10 positions were changed to Gs showed no expression in any growth condition, indicating the nucleotides replaced are required for the activity of both promoters (strain 2030). In contrast, strain 2020, in which the TA dinucleotide at −11/−10 was changed to CG, showed no expression under aerobic conditions, but the expression under anaerobic conditions was only slightly reduced (Table 1). This finding indicates that the fixRp2 promoter was drastically impaired by the double mutation, whereas it had only a moderate, if any, effect on the fixRp1 promoter. The TA dinucleotide is the only element that might conform to a canonical −10 promoter region for σ70 holoenzyme.
Table 1.
Expression of the fixR-lacZ promoter mutants
| Strain | Relevant characteristic of fixR promoter* | β-Galactosidase activity, Miller units
|
|
|---|---|---|---|
| Aerobic | Anaerobic | ||
| 8085 | Wild type | 501 ± 63 | 887 ± 8 |
| 2020 | TA → CG | 22 ± 6 | 684 ± 54 |
| 2030 | T/TA → G/GG | 3 ± 1 | 4 ± 1 |
| 2035 | TA/C/C → GG/T/G | 1,599 ± 319 | 2,389 ± 17 |
| 2068 | TA/C/C → GG/T/G | 23 ± 3 | 610 ± 54 |
See Fig. 1.
The above results are consistent with the reported extent of the −12/−24 promoters, which shows that there is no conservation downstream of position −11 (5). Thus, the fixRp2 promoter extends at least to the −10 nucleotide and therefore the fixRp1 promoter is completely within the fixRp2 sequence.
It has been documented extensively that positively regulated promoters have a weakly conserved to nonconserved −35 region, compared with promoters expressed constitutively or subject to negative control [reviewed in ref. 29]. Our proposal of the relevant nucleotides for the fixRp2 promoter suggests that fixRp2 has a poorly conserved −35 region (16), in agreement with being subject to positive regulation. To increase the similarity of the −35 region to the sequence of the glnA and fbcFH promoters, we constructed a multiple replacement at this region (Fig. 1C) and integrated it into the chromosome of the wild-type B. japonicum. This mutant form also increases the similarity of the fixRp2 promoter to the canonical −35 promoter region for σ70 holoenzyme. The resulting strain (2035) showed a marked increase in the expression of the fixR-lacZ fusion under both aerobic and anaerobic conditions (Table 1). Our interpretation of this result is that the multiple replacement generated a stronger fixRp2 promoter that is expressed at higher levels, under both aerobic and anaerobic conditions (see below). It is likely that this mutation did not affect fixRp1 because the increase in the aerobic vs. anaerobic levels of expression was similar to that in the strain with the wild-type promoter (Table 1, and see below). To evaluate whether the mutation rendered the fixRp2 promoter less dependent on positive control, the same multiple replacement was also constructed in a background carrying the A−68-to-C transversion. If the multiple nucleotide substitution resulted in a constitutive promoter the level of expression would be similar regardless of the integrity of the −68 region. Table 1 shows that the resulting strain (2068) did not express the fixR-lacZ fusion under aerobic conditions, whereas the anaerobic expression was only marginally reduced, as expected if only fixRp1 were active. Thus, we conclude that this mutant promoter, despite being highly expressed, is still dependent on the binding of the regulatory protein to the −68 region. It is likely that the multiple substitution increased the affinity of the RNA polymerase for the fixRp2 promoter. However, in vivo genomic footprinting analysis of this mutant promoter did not show any additional protection (data not shown).
Mapping of the Mutant fixRp2 Transcription Start.
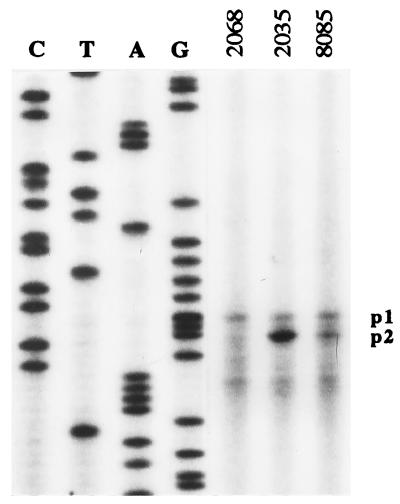
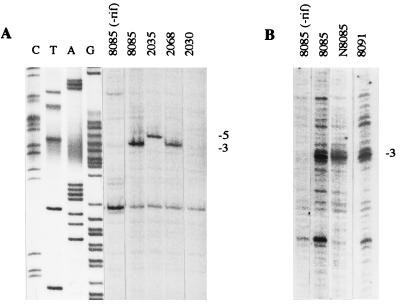
As mentioned above, the expression from each of the fixR promoters can be distinguished by the transcription start site, To corroborate that the enhanced expression of strain 2035 comes from fixRp2, total RNA from cell cultures grown anaerobically was purified and subjected to primer extension analysis. Fig. 4 shows the extension products corresponding to RNA isolated from strains 2068, 2035, and 8085. It is remarkable that the short extension product in strain 2035 is at least 3 times more intense as compared with the long product. The former product is the main transcript of fixRp2, because it is missing in the strain (2068) that has in addition the A−68-to-C mutation that renders fixRp2 inactive (16). Thus, the primer extension experiments demonstrate that the −35 mutation resulted in a more active fixRp2 promoter and did not have any effect on fixRp1. Taken together, these results strongly support our interpretation that the glnA, fbcFH, and fixRp2 promoters share functional sequence similarity.
Figure 4.
Transcription start site mapping of the −35 mutant fixRp2 mRNA. Total RNA was purified from the following strains grown anaerobically: 2068 (mutant at the −35 region in the A−68-to-C fixRnifA promoter region), 2035 (mutant at the −35 region in wild-type fixRnifA promoter region), and 8085 (wild-type fixRnifA promoter). Transcripts p1 (mainly from the fixRp1 promoter), and p2 (from the fixRp2 promoter) are indicated.
RNA Polymerase–DNA Contacts in Open Promoter Complexes.
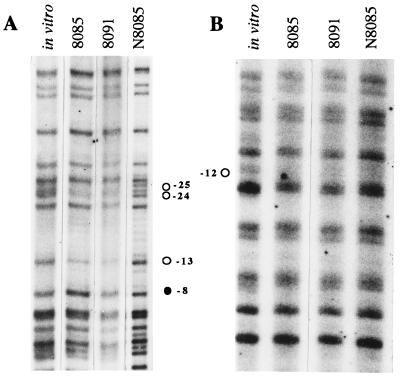
Results of primer extension (16) and of mutagenesis experiments shown above and previously published (23) suggest that the −12 and the −10 regions of the fixRp1 and fixRp2 promoters, respectively, overlap each other, whereas the −24 and the −35 regions are located one helical turn apart, as depicted in Fig. 1B (16). This implies that the two holoenzymes could interact with the same residues in the −12/−10 region. To physically localize the contact points for each RNA polymerase holoenzyme on the promoter DNA, we carried out genomic DMS footprinting experiments in B. japonicum cells previously treated with rifampicin (this antibiotic inhibits transcription elongation, resulting in accumulation of stable open complexes). In the wild-type strain residues −24, −23, and −13 of the top strand (Fig. 5A, lane 2) and −12 of the bottom strand (Fig. 5B, lane 2), the most conserved positions of the −24/−12 promoters, were protected from methylation, compared with the pattern obtained with DNA methylated in vitro in the absence of proteins (Fig. 5, lanes 1). Hypermethylation of G −8 of the top strand was also observed (Fig. 5A, lane 2). A similar protection and hypermethylation pattern has been observed at several other σ54-dependent promoters (5, 30–35). Analogous protection was observed in strain 8091 (Fig. 5, lanes 3), where the fixRp2 promoter is not active. Conversely, the protection of the top strand was not observed in strain N8085, which lacks σ54 (Fig. 5A, lane 4), indicating that the protection resulted from the interaction of Eσ54 with the promoter DNA. Interestingly, protection of G −12 of the bottom strand was detected in this strain, when compared with the DNA methylated in vitro (Fig. 5B, lane 4). This result shows that the −12 position is contacted by a holoenzyme other than Eσ54. Moreover, because the protection was also detected in the 8091 strain, which does not express the fixRp2 promoter (Fig. 5B, lane 3), and this position is protected by Eσ54 in all the −24/−12 promoters analyzed to date, it is likely that G −12 is also contacted by Eσ54.
Figure 5.
In vivo genomic DMS footprinting analysis of the B. japonicum fixRnifA promoter region. Anaerobic cultures of strains 8085, 8091, and N8085 were grown for 3 days and incubated with rifampicin before the exposure to DMS. Primer extension pattern of the fixRnifA top (A) and bottom (B) strands is shown. Control DNA methylated in vitro in the absence of proteins is shown on the left. Protected positions are indicated with ○, whereas the hypermethylated position is indicated with •.
In a previous study we unexpectedly found that the expression of fixRp2 was severalfold higher in a strain lacking σ54 than in a strain devoid of NifA. This paradoxical observation has been interpreted as the result of the formation of a stable Eσ54–fixRp1 complex that in the absence of NifA cannot proceed to clear the promoter, resulting then in a partial occlusion of fixRp2 (16). Our observation that the −12 position is contacted by both holoenzymes is consistent with a model in which both RNA polymerases compete for the binding to the same DNA, and also with the genetic data that show that disruption at the −12 position strongly affects the expression of both promoters (16, 17).
It is remarkable that guanine −12 was the only nucleotide protected by the second polymerase. This could be due to a weak interaction of this holoenzyme with other guanine residues. Alternatively, the open complex formed by this holoenzyme may not be as stable as the ones formed by Eσ54.
DNA Melting at fixRp1 and fixRp2 Promoters.
Besides the specific recognition of promoter DNA, the σ factor confers on the core RNA polymerase the ability to initiate transcription on duplex templates. This observation led to the early proposal that σ might melt the DNA by tightly binding to one DNA strand (36). Protein–DNA crosslinking studies have shown that both the β and β′ subunits of the RNA polymerase and σ70 bind to the nontemplate strand (37–39). Also, Buck and collaborators (40) have shown crosslinking of the σ54 protein with promoter DNA. deHaseth and Helmann (41) have proposed that the stabilization of single-stranded DNA in the open complex could involve interactions between σ and certain groups of the nucleotide bases not accessible on duplex DNA, such as hydrogen bonding to the nucleosides, ionic interactions with the phosphate backbone, and sequence-independent base stacking interactions with specific amino acid residues. Thus it is likely that the open complexes formed by each holoenzyme over the fixRnifA promoter region could be different.
To investigate the nature of the open complexes, anaerobic B. japonicum cultures, treated with rifampicin, were exposed to KMnO4. This single-stranded DNA selective reagent has been very useful for detecting the extent of strand separation in several prokaryotic and eukaryotic promoters (34, 42). Genomic DNA oxidized in vivo was isolated and used as template for cycling primer extension with Taq DNA polymerase, in a manner similar to that for the DMS-treated DNA. The extent of the reactive DNA in the fixRnifA promoter region is shown in Fig. 6. In the strain with the wild-type fixR promoter region, position −3 showed a marked reactivity compared with the DNA modified in the absence of rifampicin (compare lanes 1 and 2 in Fig. 6 A and B). Similar reactivity was observed when either fixRp2 or fixRp1 was the only active promoter (strains N8085 and 8091; Fig. 6B, lanes 3 and 4, respectively). This indicates that even though the two holoenzymes interact differently with the promoter DNA, and the transcription start sites are displaced by two nucleotides, the two holoenzymes form similar open complexes. Unexpectedly, when mutant 2035, which showed enhanced expression of fixRp2, was analyzed the reactivity at −3 was not observed whereas position −5 became hyperreactive (Fig. 6A, lane 3). Thus, changing the −35 promoter region resulted in the formation of a different open complex. Mutant 2068, which has the same −35 mutations in the A−68-to-C background, showed hyperreactivity at −3 (Fig. 6A, lane 4). As expected, mutant 2030, which did not show expression under any conditions, did not show sensitivity to KMnO4 (Fig. 6A, lane 5). Our interpretation of these results is that each holoenzyme generates a similar open complex; however, when fixRp2 was modified to match the consensus, and therefore expressed at higher levels, the reactivity moved upstream, indicating that a different DNA conformation was present.
Figure 6.
In vivo genomic reactivity of the fixRnifA promoter region to potassium permanganate. Anaerobic cultures were incubated with rifampicin for 10 min and exposed to KMnO4 for another 10 min, and the total DNA was purified. The first lanes of each panel show the primer extension products of DNA samples from strain 8085 that were not treated with rifampicin. (A) Effect of promoter mutations in open complex formation. Strains 8085 and 2068 showed hyperreactivity to KMnO4 of position −3, whereas in strain 2035 the hyperreactivity moved to position −5. The triple mutant strain 2030, for which no fixR-lacZ expression was detected (Table 1), did not show any hyperreactivity to KMnO4. (B) Comparison of KMnO reactivity of strains 8085, N8085, and 8091. Lanes C, T, A, and G are a sequence ladder obtained with the same oligonucleotide used for the primer extension.
The experiments presented here provide physical and genetic evidence that the fixRnifA operon is transcribed from two overlapping promoters, recognized by two different RNA polymerase holoenzymes. We showed that the mechanism of activation, promoter DNA recognition, and even melting in strain 2035 is different for each holoenzyme.
Acknowledgments
This article is dedicated to the memory of Dr. Jorge Calderón. We are grateful to F. Romero for technical assistance and to P. Gaytán and E. López for oligonucleotide synthesis. We also thank J. Aguirre, M. A. Cevallos, D. Romero, and S. López for fruitful discussions and H.-M. Fischer, X. Soberón, and B. Valderrama for their comments on the manuscript. This work was supported in part by grants from the Consejo Nacional de Ciencia y Tecnología, México (N9109-0670 and 400344-5-0132P-N). H.B. and R.G. are recipients of Consejo Nacional de Ciencia y Tecnología scholarships.
ABBREVIATIONS
- DMS
dimethyl sulfate
- UAS
upstream activator sequence
References
- 1.Kustu S, Santero E, Popham D, Keener J, Weiss D. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merrick M J. Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 3.Morett E, Segovia L. J Bacteriol. 1993;175:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer H M. Microbiol Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morett E, Buck M. J Mol Biol. 1989;210:65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- 6.Austin S, Buck M, Cannon W, Eydmann T, Dixon R. J Bacteriol. 1994;176:3460–3465. doi: 10.1128/jb.176.12.3460-3465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin S, Dixon R. EMBO J. 1992;11:2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H-s, Berger D, K, Kustu S. Proc Natl Acad Sci USA. 1993;90:2266–2270. doi: 10.1073/pnas.90.6.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- 10.Buck M, Miller S, Drummond M, Dixon R. Nature (London) 1986;320:374–378. [Google Scholar]
- 11.Morett E, Buck M. Proc Natl Acad Sci USA. 1988;85:9401–9405. doi: 10.1073/pnas.85.24.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reitzer L J, Magasanik B. Cell. 1986;45:785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- 13.Morett E, Cannon W, Buck M. Nucleic Acids Res. 1988;16:11469–11488. doi: 10.1093/nar/16.24.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Morales A, Betancourt-Alvarez M, Kaluza K, Hennecke H. Nucleic Acids Res. 1986;14:4207–4227. doi: 10.1093/nar/14.10.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubler M. Mol Microbiol. 1989;3:149–159. doi: 10.1111/j.1365-2958.1989.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 16.Barrios H, Fischer H M, Hennecke H, Morett E. J Bacteriol. 1995;177:1760–1765. doi: 10.1128/jb.177.7.1760-1765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thöny B, Anthamatten D, Hennecke H. J Bacteriol. 1989;171:4162–4169. doi: 10.1128/jb.171.8.4162-4169.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regensburger B, Hennecke H. Arch Microbiol. 1983;135:103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- 19.Fischer H-M, Alvarez-Morales A, Hennecke H. EMBO J. 1986;5:1165–1173. doi: 10.1002/j.1460-2075.1986.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer H-M. J Bacteriol. 1991;173:1125–1138. doi: 10.1128/jb.173.3.1125-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxam A M, Gilbert W. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 22.Merino E, Osuna J, Bolivar F, Soberon X. BioTechniques. 1992;12:508–510. [PubMed] [Google Scholar]
- 23.Thöny B, Fischer H-M, Anthamatten D, Bruderer T, Hennecke H. Nucleic Acids Res. 1987;15:8479–8499. doi: 10.1093/nar/15.20.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reidel G E, Brown S E, Ausubel F. J Bacteriol. 1983;153:45–56. doi: 10.1128/jb.153.1.45-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valderrama, B., Davalos, A., Girard, L., Morett, E. & Mora, J. (1996) J. Bacteriol. 178., [DOI] [PMC free article] [PubMed]
- 26.Charlton W, Cannon W, Buck M. Mol Microbiol. 1993;7:1007–1021. doi: 10.1111/j.1365-2958.1993.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 27.Beck C, Marty R, Klausli S, Hennecke H, Gottfert M. J Bacteriol. 1997;179:364–369. doi: 10.1128/jb.179.2.364-369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundig C, Beck C, Hennecke H, Gottfert M. J Bacteriol. 1995;177:5151–5154. doi: 10.1128/jb.177.17.5151-5154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClure W R. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- 30.Cannon W V, Kreutzer R, Kent H M, Morett E, Buck M. Nucleic Acids Res. 1990;18:1693–1701. doi: 10.1093/nar/18.7.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minchin S D, Austin S, Dixon R A. EMBO J. 1989;8:3491–3499. doi: 10.1002/j.1460-2075.1989.tb08514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morett E, Fischer H-M, Hennecke H. J Bacteriol. 1991;173:3478–3487. doi: 10.1128/jb.173.11.3478-3487.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popham D L, Szeto D, Keener J, Kustu S. Science. 1989;243:629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- 34.Sasse-Dwight S, Gralla J D. Proc Natl Acad Sci USA. 1988;85:8934–8938. doi: 10.1073/pnas.85.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehall S, Austin S, Dixon R. J Mol Biol. 1992;225:591–607. doi: 10.1016/0022-2836(92)90388-z. [DOI] [PubMed] [Google Scholar]
- 36.Hinkle D C, Chamberlin M. Cold Spring Harbor Symp Quant Biol. 1970;35:65–72. [Google Scholar]
- 37.Brodolin K L, Studitsky V M, Mirzabekov A D. Nucleic Acids Res. 1993;21:5748–5753. doi: 10.1093/nar/21.24.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison C A, Turner D H, Hinkle D C. Nucleic Acids Res. 1982;10:2399–2414. doi: 10.1093/nar/10.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillel Z, Wu C. Biochemistry. 1978;17:2954–2960. doi: 10.1021/bi00608a003. [DOI] [PubMed] [Google Scholar]
- 40.Buck M, Cannon W. Nature (London) 1992;358:422–424. doi: 10.1038/358422a0. [DOI] [PubMed] [Google Scholar]
- 41.deHaseth P L, Helmann J D. Mol Microbiol. 1995;16:817–824. doi: 10.1111/j.1365-2958.1995.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 42.Mirkovitch J, Darnell J E., Jr Mol Biol Cell. 1992;3:1085–1094. doi: 10.1091/mbc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]