Abstract
Antibody avidity, the strength of the multivalent interaction between antibodies and their antigens, is an important characteristic of protective immune responses. We have developed an inhibition enzyme-linked immunosorbent assay (ELISA) to measure antibody avidity for the capsular polysaccharide (PS) of Neisseria meningitidis group C (MnC) and determined the avidity constants (KDs) for 100 sera from children immunized with an MnC PS conjugate vaccine. The avidity constants were compared to the avidity indices (AI) obtained for the same sera using a chaotropic ELISA protocol. After the primary immunization series, the geometric mean (GM) KD was 674 nM and did not change in the months following immunization. However, the GM avidity did increase after the booster dose (GM KD, 414 nM 1 month after booster immunization). In contrast, the GM AI increased from an initial value of 118 after the primary immunization series to 147 6 months after the completion of the primary immunization series and then further increased to 178 after booster immunization. At the individual subject level, the avidity constant and AI correlated after the primary immunization series and after booster immunization but not prior to boosting. This work suggests that the AI, as measured by the chaotropic ELISA, in contrast to the KD, reflects changes that render antibody populations less susceptible to disruption by chaotropic agents without directly affecting the strength of the binding interactions.
Antigen-specific serum antibody titer is a reflection of both antibody concentration and affinity. While affinity is the strength of interaction between a monovalent antigen and a single antibody-binding region, the overall strength of binding is better reflected in the measurement of avidity, which is defined as the strength of the multivalent interaction between antibodies and their antigens. The equilibrium between antibody (Ab) and inhibitor (I) is described by the following equations: Ab + I ⇋ Ab·I and KD = [Ab][I]/[Ab·I], where KD is the avidity constant.
At 50% inhibition of antibody binding by competitor antigen at equilibrium, the concentration of free antibody in solution, [Ab], and the concentration of bound antibody, [Ab·I], are equal, so that KD = [I], defined as the concentration of antigen needed to inhibit antibody binding by 50% (IC50). Since tightly binding antibodies require less inhibitor to inhibit antibody binding, IC50, and therefore KD, decrease as antibody avidity increases.
Antibody avidity has been demonstrated to be an important characteristic of protective immune responses (4-7, 10, 17-19, 22, 27, 28, 31). Indeed, one of the advantages of polysaccharide conjugate vaccines over plain polysaccharide vaccines is their ability to elicit higher avidity antibodies with increased functional activity (18, 19, 22). In addition, increases in avidity are believed to correlate with B-cell memory (4-7, 17, 27, 28, 31). Thus, methods that measure avidity are useful tools in the evaluation of immune responses elicited by polysaccharide conjugate vaccines.
One common method for assessing the strength of the interaction between antibodies and antigens is a chaotropic ELISA (1-7, 10, 11, 17, 26-28, 31, 34). This protocol utilizes a chaotropic reagent, such as ammonium thiocyanate, to disrupt antibody-antigen complexes (23). The concentration of chaotrope that decreases antibody binding by 50% is defined as an avidity index (AI). McCloskey et al. demonstrated that the chaotropic ELISA ranked the antibody avidity of three monoclonal antibodies (MAbs) in the same order as affinity constants determined via biospecific interaction analysis (24).
In the late 1980s and early 1990s, inhibition ELISA protocols were developed to measure the avidity of antigen-antibody complexes (13, 25, 32). In the method reported by Friguet et al., a constant concentration of MAb is first incubated in solution with an inhibitor, and then the proportion of unbound MAb free in solution is measured by a classic indirect ELISA (13). This inhibition ELISA determines the IC50 at equilibrium, which can be converted to a KD if the concentration of inhibitor is expressed in terms of moles of epitope (13, 25, 32). The assay is sensitive, is conducted in the fluid phase, and works with very small concentrations of antibody to measure KDs in the nanomolar range (13). In addition, under conditions of total antigen excess over the total antibody concentration, the KD can be determined even with unpurified antibody preparations such as sera (13). For samples containing a mixture of antibodies with a range of avidities, such as sera, a median KD is obtained. Experimental KDs determined by inhibition ELISA protocols have been shown to be in agreement with those obtained by immunoprecipitation of radiolabeled antigen, fluorescence transfer (13), and microcalorimetry (32).
This paper describes our work to develop an inhibition ELISA to measure the median antibody avidity for the capsular polysaccharide (PS) of Neisseria meningitidis group C (MnC). We refer to the measurement of avidity rather than affinity, as the valency of the polysaccharide-antibody interaction is undefined. Two MAbs specific for the MnC PS were used as models to determine the stoichiometry per carbohydrate repeat unit (sialic acid) of MnC PS. Thus, it was possible to calculate KD from the IC50 determined via the inhibition ELISA. Using this method, median KDs were determined for several sera from adults immunized with an MnC PS conjugate vaccine. In addition, median KDs were determined for sera from 100 children immunized with an MnC PS conjugate vaccine and compared to the AIs obtained for these same sera using a chaotropic ELISA.
MATERIALS AND METHODS
Reagents.
MnC PS and methylated human serum albumin (mHSA) for the inhibition ELISA were obtained from Wyeth Vaccines Research. MnC PS and mHSA for the chaotropic ELISA were supplied by the National Institute for Biological Standards and Controls (NIBSC), Potters Bar, United Kingdom. The acetyl contents of the two MnC PS preparations were very similar: 2.24 μmol/mg and 2.12 μmol/mg for the Wyeth and NIBSC preparations, respectively. The molecular weights were determined by a combination of size exclusion chromatography and multiangle laser light scattering (MALLS; see “Microcalorimetry” for description). The MnC PS from Wyeth was a homogenous population with an average molecular mass of 3.6 × 105 g/mol. The MnC PS from NIBSC was somewhat more heterogenous, having three unresolvable molecular weight peaks, with more than 95% of the mass having a molecular mass of 1.8 × 106 g/mol.
Sera.
Sera used for developing the inhibition ELISA were collected from adults 1 month after immunization with meningococcal polysaccharide vaccine (Menomune; Aventis) (15, 16).
The pediatric sera evaluated in this study were from a completed clinical trial (30). Infants were immunized with a multivalent conjugate vaccine consisting of nine pneumococcal capsular polysaccharides (serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F, and 23F) plus MnC PS conjugated to CRM197 protein at 3, 4, and 5 months of age or at 3 and 5 months of age (primary series). Subjects were rerandomized at 12 months of age to receive booster immunizations of either the same conjugate vaccine combination or a 23-valent pneumococcal polysaccharide vaccine plus MnC PS conjugate vaccine. Sera were collected 1 month after the primary series, just prior to administration of the booster immunization (prebooster) at 12 months of age, and 1 month after the booster (postbooster). The avidity constant was determined for 100 randomly chosen subjects out of 214, with concentrations of antigen-specific immunoglobulin G (IgG) of at least 0.6 μg/ml and adequate volumes at all three time points.
Chaotropic ELISA for determining MnC PS-specific antibody avidity indices.
The chaotropic ELISA using ammonium thiocyanate for determining AIs for MnC PS-specific antibodies has been described elsewhere (17, 27). Briefly, a constant dilution of serum was allowed to react overnight with plates coated with a mixture of MnC PS and mHSA. After washing, various concentrations of the chaotropic reagent ammonium thiocyanate were added to the wells. After 15 min, the plates were washed and the remaining antibody detected. The AI is the concentration of chaotrope required to reduce the optical density (OD) by 50% compared to that in wells with no chaotrope.
Inhibition ELISA.
An inhibition ELISA was developed for human IgG specific for MnC PS to determine the concentration of MnC PS needed to inhibit 50% of antibody binding to immobilized MnC PS on a microtiter well. ELISA plates were coated with a mixture of 2.5 μg/ml MnC PS and 2.5 μg/ml mHSA in PBS. The diluent was phosphate-buffered saline (PBS) with 0.1% Brij and 0.02% NaN3 with 5% fetal calf serum. The sera were diluted to provide an OD at 450 nm of approximately 1 in wells with no inhibitor after a 2-h incubation at room temperature. An equal volume of MnC PS was added to the sera such that the final concentration of MnC PS ranged from 50 μg/ml to 0.8 μg/ml. After a 1-h incubation at room temperature, reaction mixtures were transferred to washed antigen-coated plates and incubated overnight at 4°C. Antibody was detected using goat anti-human IgG-alkaline phosphatase (Southern Biotech). The assay was developed with p-nitrophenylphosphate and stopped after 30 min with sodium hydroxide. The absorbance was read at 450 nm. For each serum, the percent inhibition was calculated for each concentration of soluble MnC PS tested by comparing the OD in wells with inhibitor to that in wells without inhibitor. For most subject sera, the inhibition was from 90% to 20% over the range of MnC PS concentrations tested. The IC50s were determined from graphs of percent inhibition versus concentration of MnC PS.
The SoftMax Pro 4.6 Enterprise edition (Molecular Devices, Sunnyvale, CA) was used for data collection and preliminary data analysis. The concentration of inhibitor required to reduce the OD by 50% was determined by plotting the percent reduction in the OD against the inhibitor concentration. The data were fitted by using the four-parameter protocol (SoftMax Pro).
Statistical analysis.
Linear regression analyses were performed to determine the relationship between avidity indices and avidity constants at each time point. One unique outlier was removed from each of the prebooster and postbooster data sets prior to the regression analysis. It was necessary to perform a Box-Cox transformation on the postbooster data set in order to build a proper regression model. The overall statistical significance of the regression models was tested using an F test (8).
MAb purification.
MAbs MNC207-3 (IgG1) and MNC46-1 (IgG1), specific for acetylated and nonacetylated epitopes, respectively (unpublished results), were generated from BALB/c mice immunized with MnC PS-CRM197 conjugates. Splenocytes were harvested from selected animals and fused to hypoxanthine-aminopterin-thymidine-sensitive nonsecreting myeloma cells using standard methodology. Resultant culture media were tested for reactivity with acetylated and deacetylated MnC PS by ELISA and were subcloned at a limiting dilution. MAbs were produced in bioreactors by Taconic (Germantown, NY). The MAbs were purified from supernatant using a BakerBond ABx column (JT Baker, Philipsburg, NJ) following the manufacturer's protocol.
Microcalorimetry.
The stoichiometry of antibody binding, or the number of saccharide repeat units per antibody-binding site on the PS can be determined from microcalorimetry experiments. Purified MAbs and MnC PS were dialyzed in the same vessel overnight at 4°C against PBS to ensure that all components were in the same buffer. The reference cell was filled with PBS. The reaction cell was filled with a purified MAb at approximately 0.25 mg/ml. The syringe was filled with MnC PS at approximately 0.25 mg/ml. The exact concentration of purified MAb used in each experiment was determined via A280. Molecular weights and concentrations of MnC PS were determined by a combination of size exclusion chromatography and MALLS. Three Synchropak SEC columns, which have nominal pore sizes of 100, 300, and 4,000 Å, were used in series. Column effluent was monitored by a variable-wavelength UV detector for protein detection, a Wyatt Technology MiniDawn multiangle laser light scattering detector, and a differential refractive index detector. Outputs from the latter two detectors were combined to calculate absolute molecular weight without use of standards or estimates. The refractive index detector response was calibrated by using dextran.
Titration experiments were performed with a VP-ITC (MicroCal LLC, Northampton, MA) at 25°C with a stirring speed of 450 rpm. In general, PS was injected into the reaction cell in 30 7-μl injections with 360 s between injections to allow the reaction cell to return to equilibrium. The heat generated by MAb binding to the MnC PS was measured for each injection, and the data were integrated to provide a titration curve. By using a nonlinear least-squares fit (MicroCal Origin version 5.0), the binding constant, the heat of binding (ΔH), and the stoichiometry of binding were extracted from the curve.
Calculation of KD.
At equilibrium, KD is ([Ab][I])/[Ab·I], where [Ab·I] is the concentration of antibody-antigen complexes, [Ab] is the concentration of uncomplexed antibody, and [I] is the concentration of uncomplexed inhibitor. At 50% inhibition at equilibrium, the concentration of antibody-inhibitor complexes is equal to the concentration of uncomplexed antibody and KD is [I], or the IC50. The IC50 can be expressed as the nanomolar concentration of antibody-binding sites added by dividing the weight-based IC50 determined from the inhibition ELISA by the product of stoichiometry of antibody binding times the molecular weight of the saccharide repeat unit.
RESULTS
Determination of stoichiometry of antibody binding to MnC PS via isothermal titration microcalorimetry.
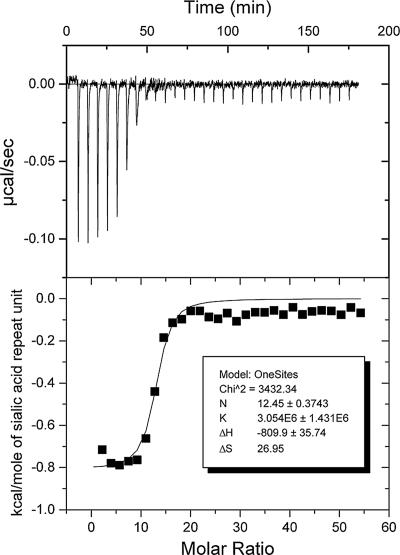
In order to calculate (median) KDs from IC50, the molar concentration of potential binding sites must be known. The stoichiometry of antibody binding, or the number of saccharide repeat units between antibody-binding sites on the PS, was determined from microcalorimetry experiments. Isothermal microcalorimetry measures changes in heat, such as that produced when an antibody binds an antigen. The heat of binding was measured by titrating known amounts of antigen into a solution of antibody. The thermodynamic binding constant, the enthalpy of binding, and stoichiometry of antibody binding, were determined from a nonlinear least-squares fit of the calorimetric titration data (9, 33). A typical titration curve is shown in Fig. 1.
FIG. 1.
Isotherm and nonlinear least-squares fit of data from isothermal titration microcalorimetry experiment with acetylated MnC PS and MAb MNC46-1, specific for deacetylated MnC PS. The average stoichiometry of binding (N), from three experiments with MAb 46-1 and two experiments with MAb MNC207-1, specific for acetylated MnC PS (data not shown), is 10.7 ± 1.5. K, thermodynamic binding constant; ΔH, enthalpy of binding; ΔS, entropy of binding.
Two MAbs, one specific for O-acetylated MnC PS and one specific for non-O-acetylated MnC PS epitopes, were used to model the stoichiometry of polyclonal antibody binding to MnC PS. For both MAbs, the stoichiometry of binding, in terms of sialic acid residues, the repeating unit of MnC PS, was determined to be 10.7. In other words, at saturation, one antibody molecule bound per 10.7 sialic acid residues along the MnC PS polymer. Thus, molar KDs were calculated from the IC50 in grams of MnC PS per liter by dividing by the molecular mass of potential binding sites, 3,116.48 g/mol, 10.7 times the molecular mass of a glycosidically linked sialic acid repeat unit (291.26 g/mol).
Reproducibility and linearity of KD determined via inhibition ELISA.
KDs determined via the inhibition ELISA were reproducible and linear. Five control sera with high, medium, or low KDs were identified from a panel of adult sera. These sera were used to determine the reproducibility of the assay (Table 1).
TABLE 1.
Reproducibility of inhibition ELISAa
| Serum source | n |
KD (nM)
|
||
|---|---|---|---|---|
| Avg | SD | CV | ||
| Adult 1 | 30 | 1,086 | 175 | 0.16 |
| Adult 2 | 27 | 930 | 144 | 0.16 |
| Adult 3 | 26 | 1.70 | 0.48 | 0.29 |
| Adult 4 | 23 | 275 | 74 | 0.27 |
| Adult 5 | 24 | 1,157 | 219 | 0.19 |
n, number of independent assays; CV, coefficient of variation.
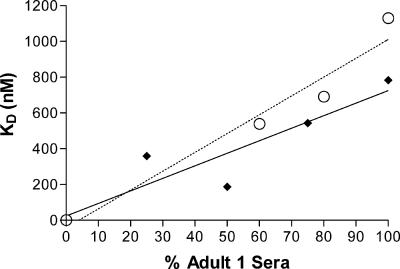
The linearity of KD determined via the inhibition ELISA was investigated by normalizing and mixing of serum from adult 1, a high-KD serum, with serum from adult 3, a low-KD serum, at known ratios (Table 1), to produce a mixed serum with a linear gradient of KDs. The results of two independent experiments are shown in Fig. 2. The KDs obtained varied linearly with the percentage of adult 1 serum in the mixed serum, suggesting that the calculated KDs represented the median avidities of the mixture of antibodies present in serum.
FIG. 2.
Linearity of inhibition ELISA. After normalizing for antibody concentration, serum from adult 1, which had a high KD, was mixed with serum from adult 3, which had a low KD, to produce a mixed serum with a linear gradient of KDs. The obtained KDs are plotted against the percentage of serum from adult 1 for each mixed serum. The results of two independent experiments are shown. Experiment 1, black diamonds and solid line (R2 = 0.83). Experiment 2, open circles and dashed line (R2 = 0.95).
Determination of KD for pediatric sera.
Avidity was determined via the inhibition ELISA for sera from 100 children immunized with the MnC PS conjugate vaccine. Sera were collected 1 month after the primary immunization series at approximately 6 months of age (post-primary series), just prior to administration of a booster immunization at 1 year of age (prebooster), and 1 month after the booster immunization (postbooster). The geometric mean concentration (GMC) of antibody post-primary series was 11.3 μg/ml. As expected, the GMC decreased over the year to 1.2 μg/ml prebooster but increased to 13.3 μg/ml postbooster. The geometric mean (GM) KDs and 95% confidence intervals (CI) for 100 subjects were 674 (631 to 721), 740 (685 to 798), and 414 (355 to 485) nM at the post-primary-series, prebooster, and postbooster time points, respectively. The GM KD remained constant from the post-primary-series to the prebooster time point (P = 0.5, t test). Less inhibitor is needed to decrease antibody binding by 50% for higher-avidity antibodies. Thus, as avidity increases, IC50 and KD decrease. The GM KD significantly decreased (i.e., the avidity increased) from the pre- to the postbooster time point (P < 0.001, t test).
Determination of AI for pediatric sera.
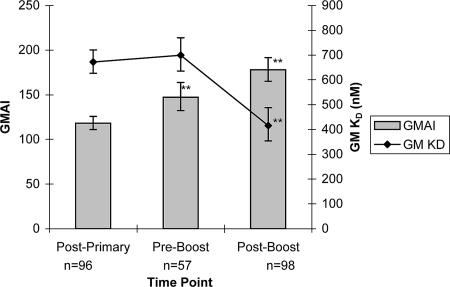
The AI was determined via the chaotropic ELISA for the pediatric sera used in the inhibition ELISA. Since a concentration of at least 0.6 μg/ml of MnC PS-specific IgG was required to determine an AI, the AI was determined for only 96, 57, and 98 post-primary-series, prebooster, and postbooster subjects, respectively. The graph in Fig. 3 illustrates that, unlike GM KD, the GM AI increased significantly from 118 post-primary series to 147 prebooster (P < 0.001, t test) and again to 178 postbooster (P < 0.001, t test). The GM KDs plotted in Fig. 3 were calculated for the subset of subjects for which the AI was determined. At all three time points, the GM KDs for these subsets were the same as for all 100 subjects tested (P > 0.05, t test): 672 nM (95% CI, 627 to 721 nM), 699 nM (95% CI, 636 to 770 nM), and 416 nM (95% CI, 354 to 488 nM) post-primary series and pre- and postbooster, respectively.
FIG. 3.
GM AI and GM KD for sera from children immunized with an MnC conjugate vaccine. Bars represent GM AI determined via chaotropic ELISA. Filled diamonds represent GM KD determined via inhibition ELISA for the same subjects. Error bars represent 95% confidence intervals for the geometric means. GM AI shows a significant increase at prebooster and postbooster time points, whereas GM KD shows a significant decrease (i.e., increased avidity) at the postbooster point only.
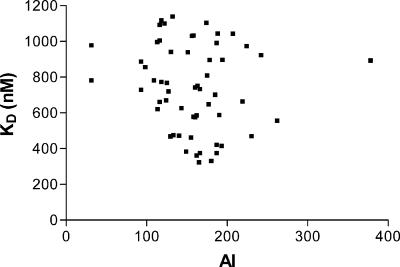
Comparing KD and AI for pediatric sera.
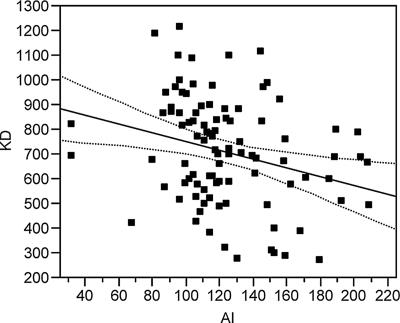
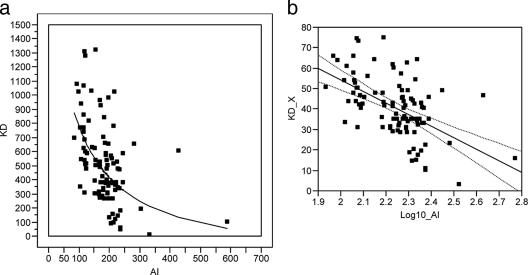
The correlation between the two measures of avidity changed over time. One month after the primary immunization series, there was a linear correlation between the two methods (R2 = 0.079, P = 0.0054) (Fig. 4). However, in the approximately 6 months leading up to the booster immunization, the AI values increased, but the KDs remained constant, and there was no linear association between KD and AI at the prebooster time point (R2 = 0.027, P = 0.1192) (Fig. 5). Following boosting, avidity measured by both techniques increased, and results derived from the two methods again showed a correlation (R2 = 0.296, P < 0.001). However, the correlation at the postbooster time point was not the same as after the primary immunization, underscoring the fact that the relationship between KD and AI varies over time. Figure 6a plots the raw data for KD and AI with an overlay of the regression model for these data (model F test: P < 0.001 from the analysis of variance [ANOVA] table, with an R2 of 0.296). The regression model between KD and AI following boosting is a curve; that is, there is a log-linear relationship between KD and AI. In order to plot the regression model as a straight line, i.e., in order to build a proper regression model, the data must be transformed as follows: KD_X = (KD)0.6 and log10(AI) equals the log-transformed values of AI (Fig. 6b).
FIG. 4.
Correlation between KD and AI after primary-series immunization. At the post-primary-series time point, KD is 929.31 − 1.79 × AI (model F test: P = 0.0054 from the ANOVA table, with an R2 of 0.08). Data points are shown as squares and the regression function as a solid line. The dashed lines around the regression line represent the 95% confidence curves for the mean of KD.
FIG. 5.
Correlation between KD and AI before booster immunization. At the prebooster time point there is no linear relationship between KD and AI.
FIG. 6.
(a) Correlation between KD and AI post-booster immunization. At the postbooster time point, the regression model between KD and AI is a curve (model F test: P < 0.001 from the ANOVA table, with an R2 of 0.296). Data points are shown as squares and the regression function as a solid line. (b) Correlation between KD and AI after booster immunization. At the postbooster time point, KD_X is 167.53 − 56.59*log10(AI), where KD_X = (KD)0.6 and log10(AI) equals the log-transformed values of AI (model F test: P < 0.001 from the ANOVA table, with an R2 of 0.296). Data points are shown as squares and the regression function as a solid line. The dashed lines around the regression line represent the 95% confidence curves for the mean of KD.
DISCUSSION
The inhibition ELISA protocol developed to determine the median KD for human IgG antibodies specific for MnC PS requires low concentrations of antibodies, approximately 0.01 μg/ml. The MnC PS-specific antibodies were first allowed to react with free PS in solution, and then the amount of unbound antibody was determined via a capture ELISA with immobilized MnC PS. The inhibition ELISA yields the IC50, in μg/ml of MnC PS.
In order to calculate KD from IC50, the molar concentration of inhibitor must be known. For monovalent inhibitors, the molar concentration of inhibitor is the same as the molar concentration of potential binding sites added. This is not the case, however, for multivalent inhibitors such as PS. Thus, the stoichiometry of antibody binding must be determined in order to calculate the molar concentration of potential binding sites added. The stoichiometry can be determined from microcalorimetry experiments (9, 12, 33). We used the binding of MnC PS-specific MAbs to model the binding of the polyclonal antibodies present in immune sera with MnC PS. We determined the stoichiometry of binding for two MAbs specific for either the O-acetylated or non-O-acetylated MnC PS to be 10.7. That is, at saturation, one IgG molecule bound per 10.7 MnC PS sialic acid residues.
The stoichiometry of antibody binding to PS appears to be determined by the steric properties of a large IgG molecule binding to the PS and obscuring the nearby epitopes, rather than by the specificity of the individual antibodies, as the stoichiometry was the same for the two MAbs with specificities for different epitopes on MnC PS. Our calculation of approximately 11 saccharides between antibody-binding events is in agreement with previously reported values (12) and our own unpublished work with six different MAbs specific for six different pneumococcal PS and three corresponding Fabs (our unpublished data). Thus, MAbs can be used to model the stoichiometry of a more polyclonal antibody population, since the stoichiometry of binding to PS appears to be influenced more by steric considerations and less by the density of epitopes along the polysaccharide chain.
The KDs for pediatric sera obtained via our inhibition ELISA for human IgG antibodies specific for MnC PS were compared to the AI determined via a chaotropic ELISA. In contrast to the inhibition ELISA, the chaotropic ELISA requires much greater concentrations of antibody, approximately 0.6 μg/ml. The MnC PS-specific antibodies were first allowed to react with immobilized MnC PS, and then chaotrope was added to disrupt antibody-antigen interactions. A consequence of the chaotropic ELISA's requirement for greater concentrations of antibodies was that the AI could be determined for only a subset of the sera for which the KD may be determined. For the work reported here, the AI could be determined for only 57 of the 100 sera at the prebooster time point due to low antibody concentration.
The values determined by the two methods behaved differently over time. The KDs remained constant from the post-primary-series point to the prebooster point before significantly decreasing postbooster (i.e., avidity increased postbooster). In contrast, the AI showed significant increases over the entire time course, suggesting that the two methods are not completely equivalent.
At the individual subject level, there was a linear correlation between the values measured by the two methods in post-primary-series sera. However, there was no correlation between the two methods in prebooster sera. This result is not surprising given the different relative changes of the two methods over the 6-month interval from the post-primary-series time point to the prebooster point. There was again a correlation between AI and KD in the postbooster sera. However, there was a log-linear relationship between KD and AI at the postbooster time point compared to the linear relationship at the post-primary series time point, showing that the relationship between these two methods varies over time.
Chaotropic reagents disrupt antibody-antigen complexes by interfering with the various interactions (e.g., electrostatic, hydrogen bond, van der Waals, hydrophobic, etc.) that maintain the complex. However, antibody conformation is maintained by some of these same forces and may be disrupted as well. Chaotrope-induced rearrangements of primary antibodies on the solid phase could expose new epitopes that may be detected by polyclonal secondary antibodies (20). Thus, the AI, as measured by a chaotropic ELISA, may not measure antibody avidity alone. It likely reflects a combination of antibody stability to the chaotrope, which is dependent upon primary and secondary protein structures, and the stability and type of the interaction between antibody and antigen. Also, the chaotropic assay was performed on a solid phase, and interactions between adjacent antibodies, such as Fc-Fc interactions that may influence the strength of binding (21), cannot be ruled out.
Several variations of the chaotropic ELISA have been developed, but the unifying principle is that higher concentrations of chaotrope are generally required to disrupt higher avidity antibody-antigen complexes. Recently, Romero-Steiner et al. compared three chaotropic ELISA protocols for determining antibody AI (29). The three protocols were as follows: (i) elution of a constant amount of bound antibody with increasing concentrations of chaotrope, (ii) binding interference of multiple serum dilutions by a single concentration of chaotrope, and (iii) elution of multiple serum dilutions by a single concentration of chaotrope. Those authors concluded that the AIs were affected by serum dilution, the heterogeneity of the antibody population, and the concentration of chaotrope (29). The different chaotropic ELISA protocols make direct comparisons of AI from various investigations difficult.
The majority of anti-MnC PS MAbs recognize acetylated or nonacetylated epitopes (14). Thus, differences in acetylation status of the MnC PS may have a greater impact than differences in molecular weight. Since the acetylation statuses of the two MnC PS preparations were very similar (2.24 μmol/mg and 2.12 μmol/mg for the Wyeth and NIBSC preparations, respectively), we do not believe that differences in the MnC PS contribute greatly to the differences observed between chaotropic and inhibition ELISAs. It is unlikely that the differences between the two methods are the result of differences between the MnC PS preparations used. Differences in molecular weight will change the ratio of end-group epitopes to internal epitopes in the two preparations. However, the overall percentage of end-group epitopes in each preparation is very small, amounting to approximately 0.4% in the NIBSC preparation and approximately 2% in the Wyeth preparation. Therefore, the contribution (or proportion) of end-group epitopes is small for both preparations.
Our results and analysis suggest that AI, as measured by chaotropic ELISA, is not a direct correlate for avidity alone. Avidity and antibody stability to chaotrope are intrinsically linked in the chaotropic ELISA. Changes in antibody sequence may affect antibody stability to chaotrope independently of changes in avidity, thus changing the relationship between AI and KD. This may partly explain why the AI but not the KD increased in the period prior to booster immunization. While there was a correlation between AI and KD 1 month after the primary immunization series and 1 month after the booster immunization, there was no correlation between AI and KD 6 months after the primary immunization series, just prior to the booster immunization.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. [DOI] [PubMed] [Google Scholar]
- 3.Anttila, M., M. Voutilainen, V. Jantti, J. Eskola, and H. Kayhty. 1999. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, R., D. Goldblatt, N. Andrews, P. Richmond, J. Southern, and E. Miller. 2001. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J. Infect. Dis. 184:377-380. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, R., D. Goldblatt, N. Andrews, J. Southern, L. Ashton, S. Deane, R. Morris, K. Cartwright, and E. Miller. 2002. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United kingdom. J. Infect. Dis. 186:1353-1357. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, R., D. Goldblatt, A. Finn, J. Southern, L. Ashton, N. Andrews, G. Lal, C. Riley, R. Rahim, K. Cartwright, G. Allan, and E. Miller. 2003. Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United Kingdom. Infect. Immun. 71:5549-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Box, G. E. P., and D. R. Cox. 1964. An analysis of transformations. J. R. Stat. Soc. B 26:211-243. [Google Scholar]
- 9.Bundle, D. R., and B. W. Sigurskjold. 1994. Determination of accurate thermodynamics of binding by titration microcalorimetry. Methods Enzymol. 247:288-305. [DOI] [PubMed] [Google Scholar]
- 10.Burrage, M., A. Robinson, R. Borrow, N. Andrews, J. Southern, J. Findlow, S. Martin, C. Thornton, D. Goldblatt, M. Corbel, D. Sesardic, K. Cartwight, P. Richmond, and E. Miller. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekstrom, N., H. Ahman, J. Verho, J. Jokinen, M. Vakevainen, T. Kilpi, and H. Kayhty. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, S. V., B. W. Sigurskjold, H. J. Jennings, J. R. Brisson, R. To, W. C. Tse, E. Altman, M. Frosch, C. Weisgerber, H. D. Kratzin, et al. 1995. Evidence for the extended helical nature of polysaccharide epitopes. The 2.8 A resolution structure and thermodynamics of ligand binding of an antigen binding fragment specific for alpha-(2→8)-polysialic acid. Biochemistry 34:6737-6744. [DOI] [PubMed] [Google Scholar]
- 13.Friguet, B., A. F. Chaffotte, L. Djavadi-Ohaniance, and M. E. Goldberg. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77:305-319. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Ojeda, P. A., M. E. Monser, L. J. Rubinstein, H. J. Jennings, and K. E. Stein. 2000. Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 68:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardina, P. C., R. E. Evans, D. J. Sikkema, D. Madore, and S. W. Hildreth. 2003. Effect of antigen coating conditions on enzyme-linked immunosorbent assay for detection of immunoglobulin G antibody to Neisseria meningitidis serogroup Y and W135 capsular polysaccharide antigens in serum. Clin. Diagn. Lab. Immunol. 10:1136-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giardina, P. C., E. Longworth, R. E. Evans-Johnson, M. L. Bessette, H. Zhang, R. Borrow, D. Madore, and P. Fernsten. 2005. Analysis of human serum immunoglobulin G against O-acetyl-positive and O-acetyl-negative serogroup W135 meningococcal capsular polysaccharide. Clin. Diagn. Lab. Immunol. 12:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldblatt, D., R. Borrow, and E. Miller. 2002. Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J. Infect. Dis. 185:397-400. [DOI] [PubMed] [Google Scholar]
- 18.Granoff, D. M., and S. L. Harris. 2004. Protective activity of group C anticapsular antibodies elicited in two-year-olds by an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr. Infect. Dis. J. 23:490-497. [DOI] [PubMed] [Google Scholar]
- 19.Granoff, D. M., A. Morgan, and J. A. Welsch. 2005. Immunogenicity of an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine in 2-year old children. Vaccine 23:4307-4314. [DOI] [PubMed] [Google Scholar]
- 20.Gray, B. M., and D. R. Shaw. 1993. Artifacts with the thiocyanate elution method for estimating relative antibody avidity. J Immunol. Methods 157:269-271. [DOI] [PubMed] [Google Scholar]
- 21.Greenspan, N. S., and L. J. Cooper. 1993. Cooperative binding by mouse IgG3 antibodies: implications for functional affinity, effector function, and isotype restriction. Springer Semin. Immunopathol. 15:275-291. [DOI] [PubMed] [Google Scholar]
- 22.Harris, S. L., W. J. King, W. Ferris, and D. M. Granoff. 2003. Age-related disparity in functional activities of human group C serum anticapsular antibodies elicited by meningococcal polysaccharide vaccine. Infect. Immun. 71:275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdonald, R. A., C. S. Hosking, and C. L. Jones. 1988. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J. Immunol. Methods 106:191-194. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey, N., M. W. Turner, and T. D. Goldblatt. 1997. Correlation between the avidity of mouse-human chimeric IgG subclass monoclonal antibodies measured by solid-phase elution ELISA and biospecific interaction analysis (BIA). J. Immunol. Methods 205:67-72. [DOI] [PubMed] [Google Scholar]
- 25.Meikle, P. J., N. M. Young, and D. R. Bundle. 1990. O-antigen biotin conjugates. Preparation and use in direct competitive enzyme immunoassays. J. Immunol. Methods 132:255-261. [DOI] [PubMed] [Google Scholar]
- 26.Pichichero, M. E., T. Voloshen, D. Zajac, and S. Passador. 1999. Avidity maturation of antibody to Haemophilus influenzae type b (Hib) after immunization with diphtheria-tetanus-acellular pertussis-Hib-hepatitis B combined vaccine in infants. J. Infect. Dis. 180:1390-1393. [DOI] [PubMed] [Google Scholar]
- 27.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 28.Richmond, P., D. Goldblatt, P. C. Fusco, J. D. Fusco, I. Heron, S. Clark, R. Borrow, and F. Michon. 1999. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine 18:641-646. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Steiner, S., P. F. Holder, P. Gomez de Leon, W. Spear, T. W. Hennessy, and G. M. Carlone. 2005. Avidity determinations for Haemophilus influenzae Type b anti-polyribosylribitol phosphate antibodies. Clin. Diagn. Lab. Immunol. 12:1029-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigurdardottir, S. T., K. Davidsdottir, V. A. Arason, O. Jonsdottir, and F. Laudat. 2005. Safety and immunogenicity of CRM197 conjugated 9-valent pneumococcal and meningococcal C combination vaccine (9vPnCMnCC) administrated in two or three primary doses in infancy, abstr. 288. Twenty-Third Annual Meeting of the European Society for Paediatric Infectious Diseases-ESPID, Valencia, Spain, 18 to 20 May, 2005.
- 31.Southern, J., S. Deane, L. Ashton, R. Borrow, D. Goldblatt, N. Andrews, P. Balmer, R. Morris, J. S. Kroll, and E. Miller. 2004. Effects of prior polysaccharide vaccination on magnitude, duration, and quality of immune responses to and safety profile of a meningococcal serogroup C tetanus toxoid conjugate vaccination in adults. Clin. Diagn. Lab. Immunol. 11:1100-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorberg, E., and D. R. Bundle. 1990. Carbohydrate-enzyme conjugates for competitive EIA. J. Immunol. Methods 132:81-89. [DOI] [PubMed] [Google Scholar]
- 33.Wiseman, T., S. Williston, J. F. Brandts, and L. N. Lin. 1989. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179:131-137. [DOI] [PubMed] [Google Scholar]
- 34.Wuorimaa, T., R. Dagan, M. Vakevainen, F. Bailleux, R. Haikala, M. Yaich, J. Eskola, and H. Kayhty. 2001. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J. Infect. Dis. 184:1211-1215. [DOI] [PubMed] [Google Scholar]